Introduction
Major depressive disorder (MDD) and bipolar disorder (BD) are leading causes of disability globally (Ferrari et al., Reference Ferrari, Stockings, Khoo, Erskine, Degenhardt, Vos and Whiteford2016; Vos et al., Reference Vos, Allen, Arora, Barber, Bhutta, Brown and Coggeshall2016). More than one third of MDD patients will not respond to first-line antidepressants (Linde et al., Reference Linde, Kriston, Rücker, Jamil, Schumann, Meissner and Schneider2015) and BD is challenging to treat, with antidepressants ineffective for most patients (Sidor & Macqueen, Reference Sidor and Macqueen2011). In recent years there has been little progress in the development of new medications for mood disorders (except perhaps for ketamine in depression; Krystal, Abdallah, Sanacora, Charney, & Duman, Reference Krystal, Abdallah, Sanacora, Charney and Duman2019) but there is currently considerable interest in the possibility of repurposing medications from other areas of medicine. Specifically, it has been suggested that certain classes of antihypertensive medication (particularly calcium-channel blockers, CCBs and angiotensin antagonists, AAs) may have a role as repurposed treatments for MDD or BD (Harrison et al., Reference Harrison, Cipriani, Harmer, Nobre, Saunders, Goodwin and Geddes2016; Harrison, Tunbridge, Dolphin, & Hall, Reference Harrison, Tunbridge, Dolphin and Hall2019; Saavedra, Reference Saavedra2017; Vian et al., Reference Vian, Pereira, Chavarria, Köhler, Stubbs, Quevedo and Fernandes2017).
To date, the evidence for repurposing antihypertensive drugs to treat MDD is limited (Chowdhury, Berk, Nelson, Wing, & Reid, Reference Chowdhury, Berk, Nelson, Wing and Reid2019; Vian et al., Reference Vian, Pereira, Chavarria, Köhler, Stubbs, Quevedo and Fernandes2017). AAs have been reported in small observational studies to be associated with better mental health outcomes (Ahola, Harjutsalo, Forsblom, & Groop, Reference Ahola, Harjutsalo, Forsblom and Groop2014; Boal et al., Reference Boal, Smith, McCallum, Muir, Touyz, Dominiczak and Padmanabhan2016; Brownstein et al., Reference Brownstein, Salagre, Köhler, Stubbs, Vian, Pereira and Fernandes2018; Johansen, Holmen, Stewart, & Bjerkeset, Reference Johansen, Holmen, Stewart and Bjerkeset2012; Nasr, Crayton, Agarwal, Wendt, & Kora, Reference Nasr, Crayton, Agarwal, Wendt and Kora2011; Williams et al., Reference Williams, Pasco, Kessing, Quirk, Fernandes and Berk2016). However, a recent large linkage study found that although initial prescriptions for AAs were associated with increased risk of depression and BD, people receiving longer-term prescriptions for AAs were not at increased risk (Kessing et al., Reference Kessing, Rytgaard, Gerds, Berk, Ekstrøm and Andersen2019a, Reference Kessing, Rytgaard, Gerds, Berk, Ekstrøm and Andersen2019b).
Since the 1980s, CCBs such as verapamil have been suggested as possible treatments for mania (Celano et al., Reference Celano, Freudenreich, Fernandez-Robles, Stern, Caro and Huffman2011). This was not supported by a review of six double-blind randomised studies and 17 observational studies which found that no evidence for efficacy of CCBs in mania (Cipriani et al., Reference Cipriani, Saunders, Attenburrow, Stefaniak, Panchal, Stockton and Harrison2016). Nonetheless, despite the relatively limited evidence base to date, CCBs remain candidates for repurposing in BD because of their biological plausibility (Cipriani et al., Reference Cipriani, Saunders, Attenburrow, Stefaniak, Panchal, Stockton and Harrison2016).
There is very little published work supporting the repurposing of other antihypertensive drug classes for MDD or BD. Case reports and some clinical trials have found that beta-blockers (BB) (particularly propranolol) may be associated with increased depressive features (Luijendijk & Koolman, Reference Luijendijk and Koolman2012; Verbeek, van Riezen, de Boer, van Melle, & de Jonge, Reference Verbeek, van Riezen, de Boer, van Melle and de Jonge2011) (although recent observational work suggests that BBs have little influence on mood disorder outcomes; Boal et al., Reference Boal, Smith, McCallum, Muir, Touyz, Dominiczak and Padmanabhan2016; Johansen et al., Reference Johansen, Holmen, Stewart and Bjerkeset2012; Ko et al., Reference Ko, Hebert, Coffey, Sedrakyan, Curtis and Krumholz2002; Luijendijk & Koolman, Reference Luijendijk and Koolman2012; Nasr et al., Reference Nasr, Crayton, Agarwal, Wendt and Kora2011; Ranchord, Spertus, Buchanan, Gosch, & Chan, Reference Ranchord, Spertus, Buchanan, Gosch and Chan2016; Verbeek et al., Reference Verbeek, van Riezen, de Boer, van Melle and de Jonge2011). It is possible that depressogenic effects are restricted to more lipophilic BB (such as propranolol) which cross the blood–brain barrier (Thiessen, Wallace, Blackburn, Wilson, & Bergman, Reference Thiessen, Wallace, Blackburn, Wilson and Bergman1990; Verbeek et al., Reference Verbeek, van Riezen, de Boer, van Melle and de Jonge2011). For other antihypertensive drugs, such as diuretics and centrally acting agents, almost no evidence of any influence on mood disorder outcomes has so far been described (Celano et al., Reference Celano, Freudenreich, Fernandez-Robles, Stern, Caro and Huffman2011; Coyne, Davis, French, & Hill, Reference Coyne, Davis, French and Hill2002; Hayes et al., Reference Hayes, Lundin, Wicks, Lewis, Wong, Osborn and Dalman2019; Huffman & Stern, Reference Huffman and Stern2007; Nasr et al., Reference Nasr, Crayton, Agarwal, Wendt and Kora2011; Williams et al., Reference Williams, Pasco, Kessing, Quirk, Fernandes and Berk2016).
Our primary goal was to use Scottish national-level routine healthcare data on over 1.8 million individuals (representing more than 6 million person-years of follow-up) to assess whether patients treated with specific classes of antihypertensive medication as monotherapy were less likely to experience new-onset mood disorder episodes.
Methods
Data sources
Within the National Services Scotland Safehaven, we created two datasets of cohorts and comparison groups by linking data from: the Community Health Index; Scottish Morbidity Records (SMR) datasets including SMR00 (Outpatient Attendance) from 1997 to 2016, SMR01 (General/Acute Inpatient and Day Case) from 1981 to 2016, and SMR04 (Mental Health Inpatient and Day Cases) from 1981 to 2016; the Prescribing Information System (PIS) from 2009 to 2016 and the National Records of Scotland death certificates from 1981 to 2016. Cohort 1 included patients treated with new-onset antihypertensive treatment (defined below) who had no previous record of mood disorder. Cohort 2 included patients with new-onset antihypertensive treatment plus a past record of mood disorder. Ethical approval for the project was obtained from the Public Benefit and Privacy Panel at National Health Services Scotland Information and Statistics division.
Time periods in which potential participants were eligible for inclusion were defined on the basis of prescriptions for antihypertensive medications, as well as prescriptions and hospital admission records for psychiatric disorders. To define the cohorts, we initially used PIS data from January 2009 to December 2016 to identify individuals who had a minimum of 4 prescriptions for antihypertensives (defined using British National Formulary (BNF) (Joint Formulary Committee, 2019) sections and paragraphs 2.2, 2.4, 2.5.5 and 2.6.2, see online supplementary Table S1 for more details on the BNF classifications) within a window of up to 12 months, preceded by 6 months of no antihypertensive treatment record. We used receiving a minimum of four or more prescriptions from a single antihypertensive treatment as an inclusion criterion because typically a single prescription would cover a period of 3 months so a minimum of four prescriptions would be required to cover a period of 1 year. The 6 months without antihypertensive treatment were to ensure that subsequent antihypertensive prescriptions were for a new treatment, rather than part of an ongoing treatment regime. Patients were then excluded from cohort 1 and included in cohort 2 if they had been prescribed psychiatric medication (indicated by BNF sections 4.1, 4.2 and 4.3) within the same window. Following that, we also excluded people from cohort 1 and included them in cohort 2 if they had been admitted to hospital for psychiatric treatment (as indicated by a clinic appointment with the general psychiatry speciality in SMR00 database, ICD10 codes F10–F48, X60–X84 and Z91.5 and ICD9 codes 290–301 and 303–305 in the SMR01 database and a record in the SMR04 databases) during the antihypertensive treatment window and for the preceding 10 years. At this stage, the number of potential cohort 1 members was 968 930 for cohort 1 (see online supplementary Fig. S1), and for potential cohort 2 members this number was 555 975 (see online supplementary Fig. S2).
Given that the pool of people for the comparison group was limited, for both cohorts we selected individuals who had an eligible period that ended after 31/12/2009. We aimed to match cohort members with comparisons using a 1:1 ratio for cohort 1 and 1:2 for cohort 2. Comparisons for each cohort were initially selected on the criteria that they had received no antihypertensive medication between 2009 and 2016, and then on the same criteria as their corresponding cohort with respect to psychiatric treatment. Matching was on the basis of age (+/− 2 years), sex and Scottish index of multiple deprivation. Subsequently, cohort-comparison pairs were excluded from analysis if they either had a death record prior to the end of their eligible treatment window, or were outside the age range of 18 to 100. This resulted in 538 789 cohort-comparison pairs for cohort 1, and 272 278 cohort 2 members matched to 502 937 comparators. Descriptive statistics for unmatched but otherwise eligible patients are shown in online supplementary file Table S2 for Cohort 1 and online Supplementary Table S3 for Cohort 2. The main barrier to matching cohort members was age, with it being much harder to find matches for older cohort members particularly for cohort 2 for which we used a higher matching ratio.
Antihypertensive monotherapy and polytherapy status
Participants were identified for treatment on the basis of prescribing records for antihypertensive medication within the 2009–2016 PIS dataset. These codes were then used to classify participants into specific classes on the basis of the prescription of thiazide diuretics, BB, AAs (including angiotensin-converting enzyme (ACE)-inhibitors, angiotensin receptor blockers (ARB) and renin inhibitors) and CCBs. Patients were included in antihypertensive monotherapy groups if during the 12 months prior to the end date of the eligibility period they received four or more prescriptions from a single class of antihypertensive treatment and no prescriptions for any of the other classes. For the analysis of monotherapy, individuals were subsequently censored at the date on which they received a prescription for an antihypertensive drug outside their monotherapy class. Patients were considered to be on polytherapy if during the last 3 months of the eligible treatment window they received antihypertensive medication from two or more of thiazide diuretics, BBs, AA or CCBs. Remaining study participants who had received at least four antihypertensive treatments but were not eligible for the monotherapy or polytherapy groups were classified as ‘other antihypertensive’. See online Table S4 in the supplementary file for the frequency of each of the different combinations.
Outcome measures
We had two outcome measures indicating new-onset of treatment for episodes of either MDD or BD, indicated by receipt of medications (PIS) or psychiatric admissions (SMR04) subsequent to the end date of the eligibility window used to define the cohorts. Using PIS data, new onset of treatment for MDD was identified by the prescription of any antidepressant drugs (BNF section 4.3), and new onset of BD was identified by a prescription for antipsychotics and drugs used for mania and hypomania (BNF section 4.2). Similarly, the ‘main’ and ‘other diagnoses’ fields in SMR04 were used to identify first episodes for treatment for MDD or BD (ICD10 codes F32 and F33 used to indicate MDD and ICD10 codes F30 and F31 used to indicate BD).
Confounding variables
Medical comorbidities for study members were defined by having ever received specific prescriptions or attended hospital for specific conditions as indicated in available PIS, SMR00, SMR01 and SMR04 records, up to end date of the eligibility window.
Using PIS data, ever prescribed cardiovascular medication (other than antihypertensives) was defined using prescriptions for drugs from BNF sections or paragraphs 2.1, 2.3, 2.6.1, 2.6.3, 2.6.4, 2.9 and 2.12, and treatment for diabetes was indicated by receipt of prescriptions of antidiabetic medications (BNF chapter 6.1.2.)
Confounding variables were derived from hospital records based on specific ICD10 and ICD9 codes, as indicated in the ‘main’ and ‘other conditions’ for SMR00, SMR01 and SMR04 and ‘main’ admission and ‘other’ admission condition for SMR04. History of cardiovascular disease was defined using ICD10 codes I20–I25 (ischaemic heart diseases), and I60–I69 (cerebrovascular diseases), and ICD9 codes 410–414 (acute myocardial infarction) and 430–438 (cerebrovascular diseases). Head injuries were identified using ICD 10 codes S02.0, S02.1, S02.7, S06, S07 and ICD9 codes 800–804 and 850–854. Substance abuse was identified using ICD codes F10 to F19, and ICD9 codes 291, 292, 303 to 305. Self-harm was identified using ICD codes X60–X84 and Z91.5 and ICD 9 codes E950–E958.
Additional confounding variables were defined for cohort 2 who had a history mental illness or mood disorders. PIS data was used to identify people who had been prescribed the following types of pharmaceutical treatment: hypnotics or anxiolytics (BNF Section 4.1), antidepressants (BNF Section 4.3), or drugs used in psychoses and related disorders (BNF Section 4.2). Hospital admission records were used to identify people who had been admitted to hospital for the following conditions: Schizophrenia and delusional disorders (ICD10 codes F10–F19 and ICD9 codes 291–292 and 303–305), MDD (ICD10 codes F32–F33, and ICD9 codes 296.2–296.3), BD (ICD10 codes F30–F32, and ICD9 codes 296.0, 296.1 and 296.9) and personality disorders (ICD10 codes F60–F69, and ICD9 code 301).
Data analysis
All analyses were carried out within the National Safehaven using Stata 14.0 MP. The curves for first onset of mood disorder by therapy classes are presented using cumulative distributive functions, which are calculated as 100 percent minus the Kaplan–Meier estimate (Cleves, Gould, & Marchenko, Reference Cleves, Gould and Marchenko2016). Data were analysed using Cox proportional hazards models, which were stratified by cohort and control pairs, to investigate the relationship between specific therapy classes and new-onset of MDD or BD following the end of the 12 month eligibility window, after adjustment for age and the other medication and hospital admission variables. Preliminary analyses suggested that the proportional hazards assumption was violated; consequently this was addressed using a Heaviside function (Kleinbaum & Klein, Reference Kleinbaum and Klein2010) with separate hazard ratios (HRs) calculated for the specific therapy classes for the following five time periods: 0 to 3 months, >3 to 6 months, >6 to 12 months, >1 year to 2 years and >2 years.
Results
Sociodemographic characteristics for cohort 1 are shown in Table 1, and a comparison of both cohorts is shown in online Supplementary Table S5. The mean age of the different antihypertensive treatment groups varied, with patients on BBs having the youngest mean age of 53.4 years, while those in the ‘other antihypertensive’ group had the oldest mean age of 66.6 years. Patients receiving thiazide diuretics, BBs and other patterns of antihypertensive treatments were more likely to be women, while those receiving AAs, CCBs and polytherapy were more likely to be men. There were small differences between the groups in terms of area deprivation, with patients receiving thiazides the most affluent and those on other medications the least affluent.
Table 1. Sociodemographic, medical event and history variables Cohort 1
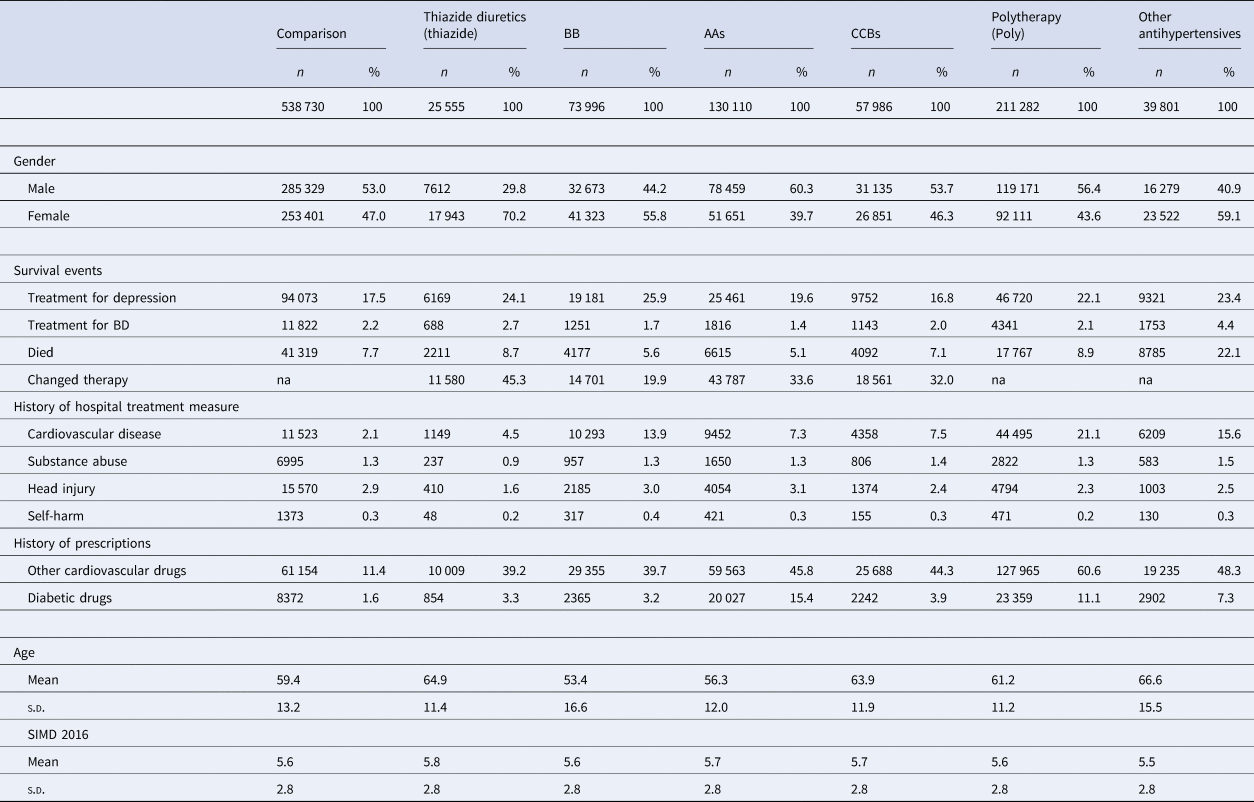
‘Other Antihypertensives’ were defined on the basis of treatment with a combination of thiazide diuretics, diuretics, BBs, AA and/or CCBs, but not treatment with at least two of these groups within in the last 3 months of the eligible treatment window.
Medical history and mood disorder outcomes are also shown in Table 1 for cohort 1 and in online Supplementary Table S6 for cohort 2. As might be expected for the comparison group, both the percentages of people having been admitted to hospital for cardiovascular disease and receiving prescriptions for other cardiovascular medicines were lower than the equivalent figures for all the antihypertensive treatment groups. However, for the other medical history measures the comparison groups fell within the range of the antihypertensive treatment groups.
Cumulative distribution functions
The cumulative distribution functions for new-onset of an episode of MDD by therapy class are shown in Fig. 1a for cohort 1 (see online Supplementary Fig. S3a for cohort 2). Numbers at risk and number of failures for both the treatment cohorts are shown in online Supplementary Table S7. The main difference between the two cohorts was the expected higher incidence rates of MDD episodes in the first 0–3 months for cohort 2, reflecting that (by definition) these patients already had a record of mood disorder. The comparison groups for both cohorts had the lowest risk of receiving treatment for MDD throughout the follow-up period, and patients receiving BB monotherapy had the highest risk for MDD episodes. The curve for the ‘other antihypertensive’ treatment group in cohort 1 was similar to the curve for those receiving BBs in that cohort. Among all the antihypertensive monotherapy groups, those receiving AAs had the lowest risk of new-onset MDD.
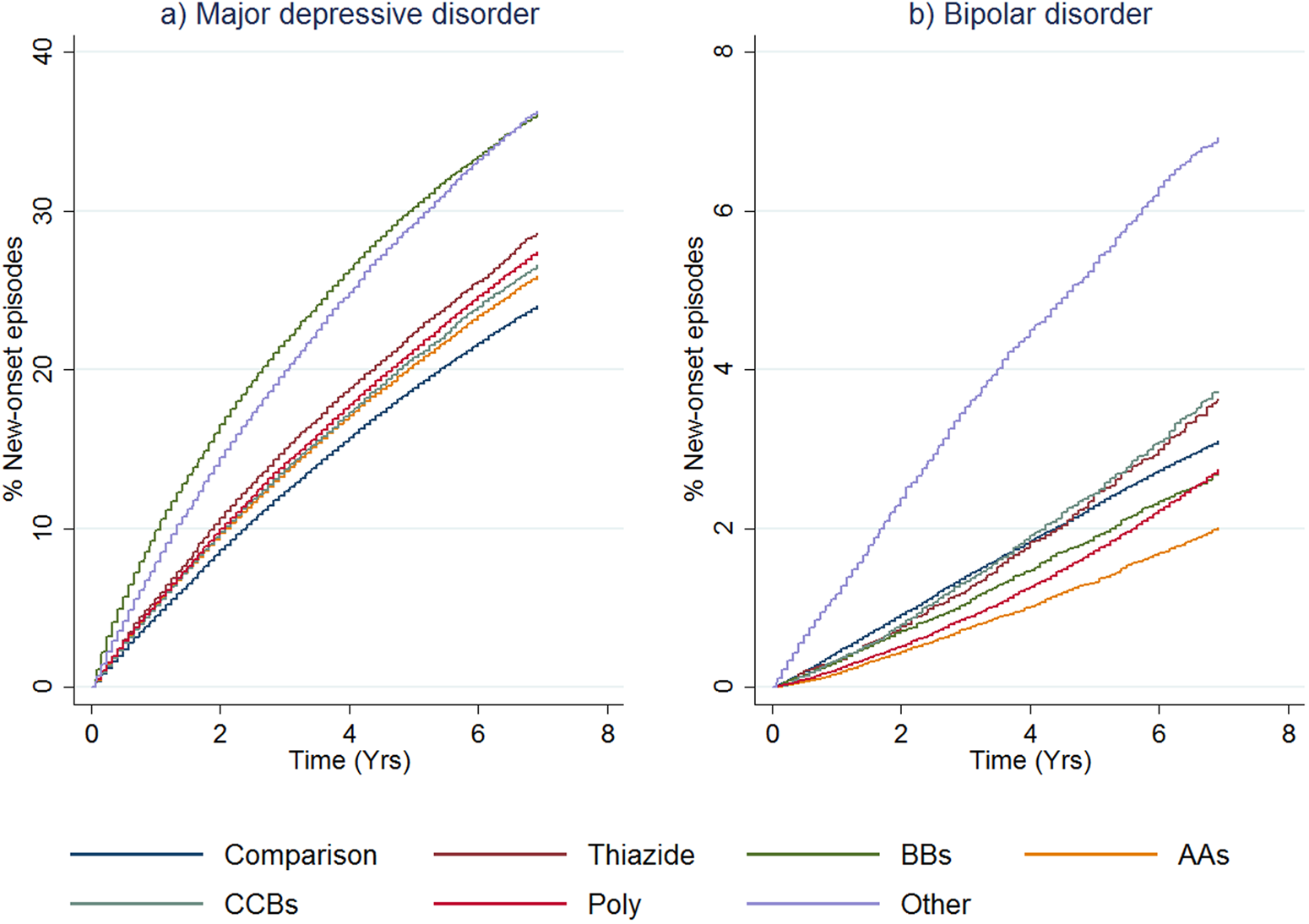
Fig. 1. First onset of mood disorders, as indicated by receipt of prescriptions or admission to hospital, by therapy class (people without mental illness). This figure shows cumulative distribution functions for MDD (panel a) and BD (panel b) by therapy class for people without a history of treatment for mental illness (cohort 1). ‘Other Antihypertensives’ were defined on the basis of treatment with a combination of thiazide diuretics, diuretics, BBs, AA and/or CCBs, but not treatment with at least two of these groups within in the last 3 months of the eligible treatment window.
The cumulative distribution functions for new-onset BD episodes by the antihypertensive monotherapy group are shown in Fig. 1b. The equivalent figure for cohort 2 is shown in online Supplementary Fig. S3b. For cohort 1, the ‘other antihypertensive’ group was more likely to receive treatment for new-onset BD compared to all the other classes of treatment. The comparison group curve lay between the curves for the other antihypertensive classes, and people receiving polytherapy and AAs were somewhat less likely to be treated for BD than the other therapy classes. Once the (expected) sharp incidence rate is accounted for in cohort 2, curves were similar to cohort 1.
Cox proportional hazard models for new-onset of depression
The HRs (after adjustment for hospital treatment for cardiovascular disease, substance abuse, head injury, self-harm and pharmaceutical treatment for other cardiovascular or diabetic drugs) for the relationship between receiving a specific class of antihypertensive and new-onset MDD over time for people without a prior history of mood disorder (cohort 1) are shown in Fig. 2.
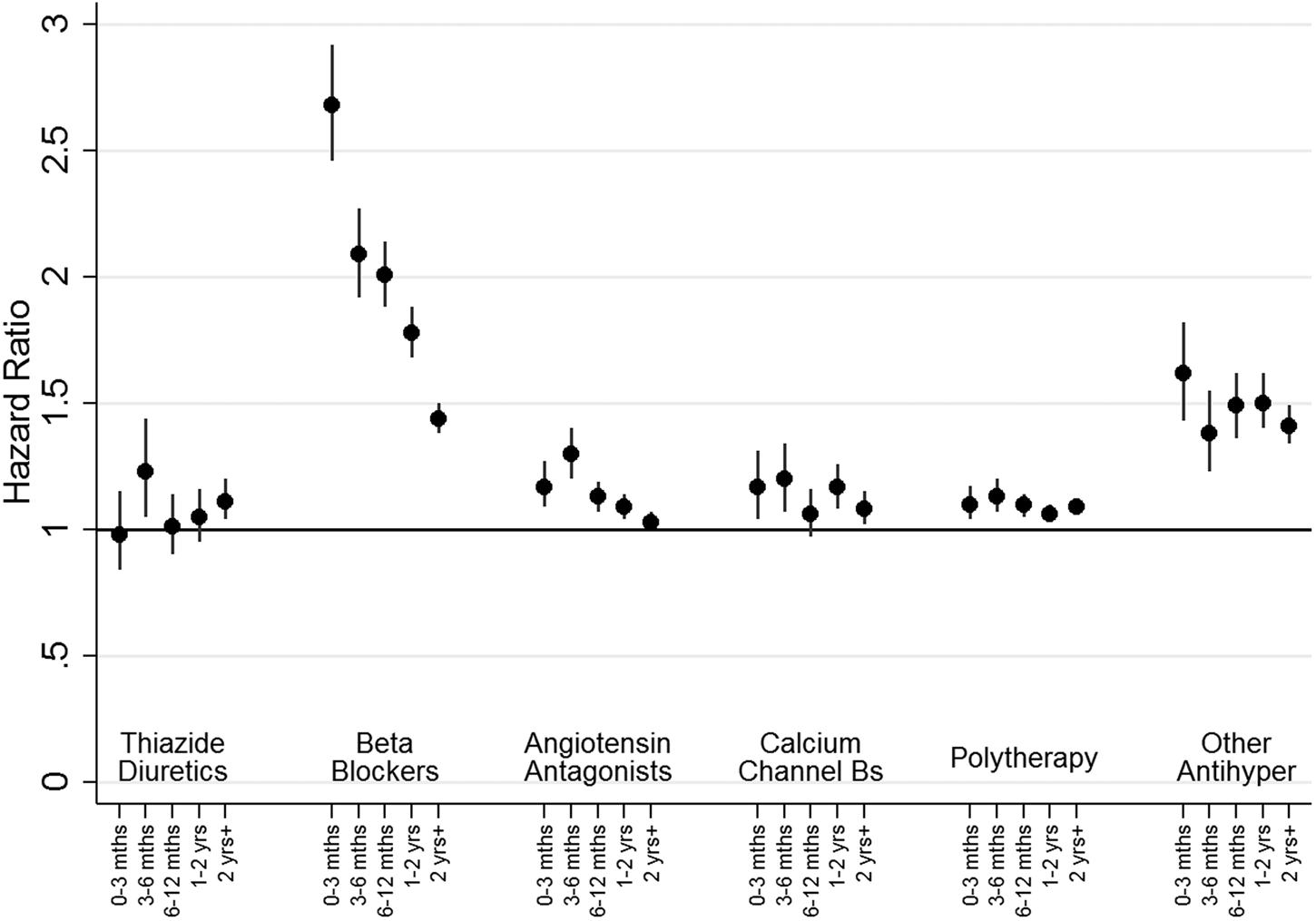
Fig. 2. HRs for new onset depression, as indicated by receipt of prescriptions or admission to hospital, by therapy class (people without mental illness). This figure shows HRs for new onset depression by therapy class for people without a history of treatment for mental illness (Cohort 1). Adjustment was carried out for hospital treatment for cardiovascular disease, substance abuse, head injury, self-harm and pharmaceutical treatment for other cardiovascular drugs and diabetic drugs. ‘Other Antihypertensives’ were defined on the basis of treatment with a combination of thiazide diuretics, diuretics, BBs, AA and/or CCBs, but not treatment with at least two of these groups within in the last 3 months of the eligible treatment window.
For people receiving most monotherapy treatments and the polytherapy group there was a small but consistently elevated HR of around 1.2, which declined with time. For people receiving ‘other antihypertensive’ treatments, there was a slightly higher HR of around 1.5 for all time points. In contrast, the HR for people treated with BBs was initially high at 2.68 (95% CI 2.45–2.92) in the first 3 months, before declining to 2.01 (95% CI 1.88–2.13) after 6 months and 1.44 (95% CI 1.38–1.50) at 2 years.
We explored the possibility that any associations were restricted to sub-groups among AAs and BBs. During the first 3 months the HR for participants being treated with propranolol (N = 30 478) was 4.80 (95% CI 4.22–5.46), with this falling over time (3 to 6 months: HR 3.74, 95% CI 3.31–4.23, 6 to 12 months: HR 3.29, 95% CI 2.99–3.62, 1 to 2 years: HR 2.73, 95% CI 2.51–2.97) to just 2.04 (95% CI 1.91–2.17) after 2 years. In contrast, the associations for atenolol (n = 16 650), other BB (N = 26 868) and the two subclasses of AAs (ACE inhibitors, N = 105 064, or ARB, N = 16 071) were consistently weak, with a HR of around 1.1. We also ran additional analyses separately for men (online Supplementary Fig. S4) and women (online Supplementary Fig. S5). The odds ratios for new-onset depression for nearly all antihypertensive classes were larger for women than the corresponding odds ratios for men, however, the gender differences were small and conclusions drawn for both genders are similar.
There was greater consistency of relationships between all therapy classes of antihypertensive treatment and new-onset MDD episodes for people with a prior history of mood disorder (cohort 2; online Supplementary Fig. S6). For most antihypertensive groups, the HRs were around 1.4 at 0–3 months and about 3 for between 3–6 months, with HRs continuing to increase thereafter. However, BBs appeared to have a slightly stronger association at 0 to 3 months (HR 1.93, 95% CI 1.90–1.96) and at 3 to 6 months (HR 3.83, 95% CI 3.63–3.04). Further analyses indicated that elevated HRs for people on BBs were restricted to people who had been prescribed propranolol.
Cox proportional hazard models for new-onset bipolar disorder
In adjusted analyses for people with no previous history of mood disorder (cohort 1), most therapy classes of antihypertensive drugs were initially associated with reduced risk of BD episodes, with the risk subsequently increasing towards a null association over time (see Fig. 3). The exceptions were those on BBs and other treatment groups, for whom there appeared to be some limited evidence of association with BD outcomes. As before, we investigated subgroups within AA and BBs. The associations for both ACE inhibitors and ARBs were the same as for AAs combined, and participants who were treated with atenolol and other BBs also had a reduced risk of being treated for BD. In contrast, those treated with propranolol had a much higher risk of being treated for BD at 0–3 months (HR 3.33, 95% CI 1.08–10.27), with the risk falling gradually thereafter. We ran separate analyses for men (online Supplementary Fig. S7) and women (online Supplementary Fig. S8) and there was little evidence of gender differences that could not have occurred by chance.
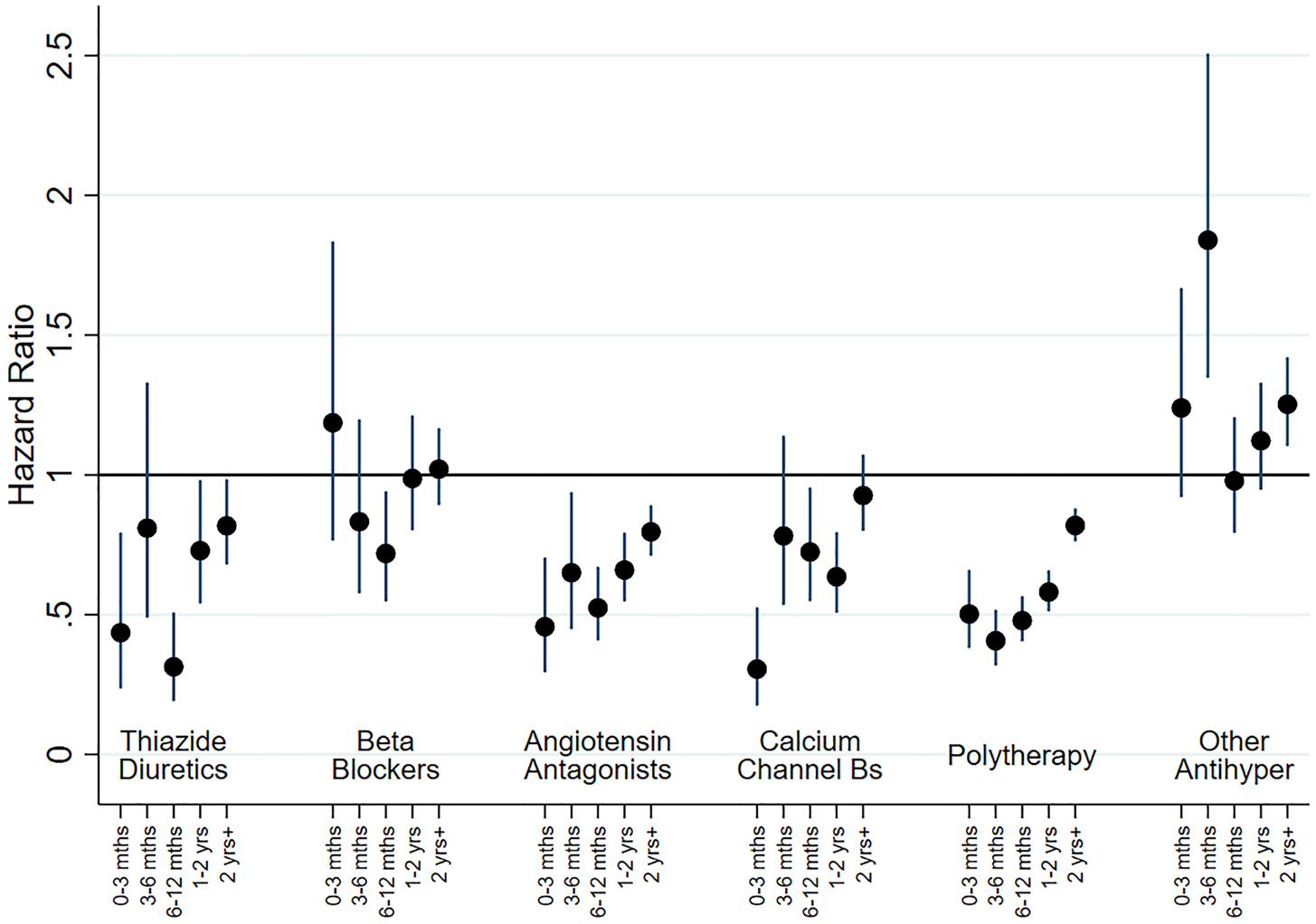
Fig. 3. HRs for new onset BD, as indicated by receipt of prescriptions or admission to hospital, by therapy class (people without mental illness). This figure shows HRs for new BD by therapy class for people without a history of treatment for mental illness (Cohort 1). Adjustment was carried out for hospital treatment for cardiovascular disease, substance abuse, head injury, self-harm and pharmaceutical treatment for other cardiovascular drugs and diabetic drugs. ‘Other Antihypertensives’ were defined on the basis of treatment with a combination of thiazide diuretics, diuretics, BBs, AA and/or CCBs, but not treatment with at least two of these groups within in the last 3 months of the eligible treatment window.
A slightly more complex pattern was evident in adjusted analyses for those with a prior history of mood disorder (cohort 2; online Supplementary Fig. S9). Most antihypertensive classes were initially associated with a small reduction in risk during the first 3 months. However, this tended to change over time and after a year was associated with increased risk of BD. The exceptions to this were the ‘other antihypertensive’ group and BBs, which were both associated with increased risk of new-onset treatment for BD. As above, additional analyses indicated that this elevated risk for BBs was restricted to propranolol.
Discussion
Overall, our findings do not provide support for the repurposing of antihypertensive drugs as treatments for depression. Relative to the comparison group, most classes of antihypertensive were associated with small increased risks of being treated for MDD and a slightly lower risk of being treated for new-onset BD. The main exceptions to this were for patients treated with BBs, which were associated with increased risk of subsequent treatment for both MDD and BD (this is perhaps unsurprising given the widespread use of BBs as anxiolytics).
Our results are consistent with studies suggesting that propranolol may be associated with increased long-term risk of mood disorders (Luijendijk & Koolman, Reference Luijendijk and Koolman2012; Verbeek et al., Reference Verbeek, van Riezen, de Boer, van Melle and de Jonge2011). Additionally, given our study design, our findings are consistent with those of the work of Kessing and colleagues (Kessing et al., Reference Kessing, Rytgaard, Gerds, Berk, Ekstrøm and Andersen2019a, Reference Kessing, Rytgaard, Gerds, Berk, Ekstrøm and Andersen2019b). Relative to untreated comparison group's, both Kessing et al. and our study had similar results in that prescriptions for AAs were associated with a small increased risk of depression (Kessing et al., Reference Kessing, Rytgaard, Gerds, Berk, Ekstrøm and Andersen2019b) and a reduced risk of BD (Kessing et al., Reference Kessing, Rytgaard, Gerds, Berk, Ekstrøm and Andersen2019a).
Our design is an advance on previous work (Boal et al., Reference Boal, Smith, McCallum, Muir, Touyz, Dominiczak and Padmanabhan2016) which did not include a control group. Kessing et al. correctly highlight that people with hypertension have a small increased risk of future depression. Their solution was to compare periods where patients had received a cumulative three or more prescriptions of AAs relative to a reference period which included people who had only one or two prescriptions for AAs. While this approach might to some extent address the confounding effects of hypertension, it does also have a potential to add other biases. The comparison group used by Kessing et al. included people who only ever received one or two prescriptions of AAs. In our study, those who received an initial treatment with AAs but then swapped to other antihypertensives had greatly elevated risk of depression, suggesting that the control group in the Kessing et al. study may be confounded by other aspects relating to adherence to medication (Kessing et al., Reference Kessing, Rytgaard, Gerds, Berk, Ekstrøm and Andersen2019a, Reference Kessing, Rytgaard, Gerds, Berk, Ekstrøm and Andersen2019b).
Using observational data it is extremely unlikely that any one study design will address all potential biases, and it is necessary to compare across studies and designs, a process termed triangulation (Lawlor, Tilling, & Davey Smith, Reference Lawlor, Tilling and Davey Smith2016). On balance, it would appear from the existing literature that AAs are a good candidate for repurposing to treat BD. The evidence for repurposing antihypertensive drugs to treat MDD is weaker. Previously reported protective associations (Kessing et al., Reference Kessing, Rytgaard, Gerds, Berk, Ekstrøm and Andersen2019b) and the negative associations in our study are weak and occur across different classes of medication. The similarity of associations across multiple classes of medication might reflect non-biological mechanisms and it is well established that patients who are in regular contact with their General Practitioners (for example, for medication reviews or monitoring of blood pressure) are more likely to have psychological problems identified than patients with fewer regular consultations (Bushnell, Reference Bushnell2004). Ultimately only a well-designed randomized controlled trial is likely to resolve these issues but it is unclear at this stage whether the opportunity costs in carrying out such a study are justified.
Limitations
In 2011, which is within our study period, guidelines on the treatment of hypertension changed and BBs were no longer a preferred initial therapy for hypertension (National Institute for Health & Care Excellence (NICE), 2011). As noted above, BBs such as propranolol are commonly used in primary care settings to treat anxiety (Hayes & Schulz, Reference Hayes and Schulz1987), as well as for thyrotoxicosis, and angina. It was not possible for us to identify from prescription records the exact reasons for treatment choices. The observed increased risk of new-onset MDD in this group might therefore reflect the exacerbation of an already-established affective disorder.
The comparison groups clearly differ with respect to cardiovascular history and, despite the cohorts and comparison groups being selected on the basis of their mood disorder histories, they may also differ with respect to subclinical symptoms of anxiety and depression. However, for cohort 1 these differences were likely to be small for all antihypertensive classes, except BBs. In contrast, cohort 2's risk of mood disorders (relative to their comparison group) continued to increase over time. This could reflect poorer mental health among cohort 2, undiagnosed onset of new mental illnesses among the comparison group, or residual confounding factors. Potentially eligible participants for whom we could not find matches differed from those analysed in that they were older and tended to have poorer health.
New-onset BD was primarily identified using PIS records. As such, it was not possible to distinguish between bipolar depressive states and hypomanic or manic states based on the data that we had access to. In addition, given that most cases of BD tend to have an onset in early adulthood (many years before treatment for hypertension usually starts), it is not clear that we have accurately captured new-onset BD. It is also possible that some of these patients may have received treatment for BD earlier in life, during periods (before 1981 for SMR04 and before 2009 for PIS) for which data was not available.
Our study design identified people who consistently received antihypertensive monotherapy in order to investigate possible protective effects of antihypertensive treatments in the medium- to long-term. As such, this study was not designed to investigate immediate side effects of antihypertensive treatments. A patient who developed depressive symptoms and received treatment for MDD quickly after their first antihypertensive medication prescription would, by design, be included within cohort 2. However, it is difficult to distinguish such people from those who were selected into cohort 2 due to having prior mental illness. Consequently, for cohort 2, which we considered our secondary analyses, the more interesting aspects of the results are for periods occurring at least 6 months after the initial period of eligibility. A patient who changed their antihypertensive treatments in the first 12 months of treatment, perhaps due to side-effects, would have been included in the ‘other antihypertensive’ group, such that membership of this group may indicate additional health problems beyond hypertension. We also did not have data on whether or not hypertension was being adequately controlled by medication. This could potentially confound the results. Another challenge was the classification of patients treated with multiple antihypertensive treatments. We used a pragmatic measure of whether people had received treatment from at least two antihypertensive classes within the final 3 months of the window used to define eligibility. In theory, this might include people on changing prescription regimes. However, the risks of MDD and BD for people in the polytherapy group were within the range of most monotherapies, suggesting that this was a reasonably robust approach.
Conclusion
Using a comprehensive national-level routine healthcare data linkage approach, we found little evidence to support the idea that antihypertensive medications might be usefully repurposed as treatments for MDD. Tentatively, we conclude that some classes of antihypertensive – and AAs in particular – may offer some protection against BD, but this could be due to selection biases, and will require other study designs to resolve.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291719004094
Acknowledgements
The authors would like to acknowledge the support of the eDRIS Team (National Services Scotland) for their involvement in obtaining approvals, provisioning and linking data and the use of the secure analytical platform within the National Safe Haven.
Financial support
This project was supported by The Chief Scientist Office of Scotland award (D.J.S, D.M, J.P.P, S.P., grant number TCS/16/06) and the UK MRC Mental Health Data Pathfinder Award (D.J.S., J.P.P., grant number MC_PC_17217).
Conflict of interest
None.







