Introduction
Non-suicidal self-injury (NSSI) is the deliberate, self-directed damage of body tissue without suicidal intent and for purposes not socially or culturally sanctioned (Klonsky, Victor, & Saffer, Reference Klonsky, Victor and Saffer2014). NSSI typically manifests during adolescence (Plener, Schumacher, Munz, & Groschwitz, Reference Plener, Schumacher, Munz and Groschwitz2015) and is often observed in major depressive disorder (MDD) (Weissman et al., Reference Weissman, Wickramaratne, Nomura, Warner, Pilowsky and Verdeli2006). Our previous research, as well as that of others, has found that approximately 40–60% of adolescent patients with MDD have a history of NSSI (Asarnow et al., Reference Asarnow, Porta, Spirito, Emslie, Clarke, Wagner and Brent2011; Hu et al., Reference Hu, Huang, Shang, Huang, Jiang and Yuan2023; Wang, Liu, Yang, & Zou, Reference Wang, Liu, Yang and Zou2021). In addition, NSSI is an important risk factor for suicidality (Koenig et al., Reference Koenig, Brunner, Fischer-Waldschmidt, Parzer, Plener, Park and Kaess2017), highlighting the importance of clinical intervention. Although the psychological and social factors contributing to the risk of NSSI are well understood, the biological mechanisms are only beginning to be uncovered. Neurobiological studies of NSSI have focused on genetics (Hankin, Barrocas, Young, Haberstick, & Smolen, Reference Hankin, Barrocas, Young, Haberstick and Smolen2015), neuroendocrine systems (including the HPA axis and the endogenous opioid system) (Klimes-Dougan et al., Reference Klimes-Dougan, Begnel, Almy, Thai, Schreiner and Cullen2019; Störkel et al., Reference Störkel, Karabatsiakis, Hepp, Kolassa, Schmahl and Niedtfeld2021), and neuroimaging (including brain structure, connectivity, and behavioral indicators of brain functioning), with neuroimaging being a particularly active area of research. The dysfunction in frontolimbic circuits proposed by neuroimaging studies is particularly noteworthy (Auerbach, Pagliaccio, Allison, Alqueza, & Alonso, Reference Auerbach, Pagliaccio, Allison, Alqueza and Alonso2021; Kaess et al., Reference Kaess, Hooley, Klimes-Dougan, Koenig, Plener, Reichl and Cullen2021). This dysfunction is associated with difficulties with emotion regulation (DER) (Kohn et al., Reference Kohn, Eickhoff, Scheller, Laird, Fox and Habel2014; Wilcox, Pommy, & Adinoff, Reference Wilcox, Pommy and Adinoff2016) and may contribute to the development of NSSI and depression psychopathology (Auerbach et al., Reference Auerbach, Pagliaccio, Allison, Alqueza and Alonso2021; Westlund Schreiner et al., Reference Westlund Schreiner, Mueller, Klimes-Dougan, Begnel, Fiecas, Hill and Cullen2020).
The frontolimbic circuit, a neural network critical for emotion regulation and processing, comprises multiple prefrontal subregions, including the ventrolateral prefrontal cortex (PFC) and anterior cingulate cortex (ACC), as well as key limbic structures such as the amygdala, hippocampus, and striatum (Lindquist, Satpute, Wager, Weber, & Barrett, Reference Lindquist, Satpute, Wager, Weber and Barrett2016; Phillips et al., Reference Phillips, Chase, Sheline, Etkin, Almeida, Deckersbach and Trivedi2015). Empirical evidence suggests that this circuit is characterized by a functional imbalance both between and within its constituent components (Bhatia, Henderson, Hsu, & Yim, Reference Bhatia, Henderson, Hsu and Yim2018; Phillips, Drevets, Rauch, & Lane, Reference Phillips, Drevets, Rauch and Lane2003), which may be partly attributed to alterations in the microstructure of white matter (WM) bundles, particularly those located in the cingulum (CB) that provide interconnections among critical nodes of the frontolimbic system (Bhatia et al., Reference Bhatia, Henderson, Hsu and Yim2018; Westlund Schreiner et al., Reference Westlund Schreiner, Mueller, Klimes-Dougan, Begnel, Fiecas, Hill and Cullen2020).
The cingulum bundle (CB) is a crucial white matter tract surrounding the corpus callosum. It interconnects the ipsilateral subcortical nuclei, the cingulate gyrus, and several areas of the frontal, medial temporal, and parietal lobes (Bubb, Metzler-Baddeley, & Aggleton, Reference Bubb, Metzler-Baddeley and Aggleton2018). Its contribution spans diverse cognitive domains, including emotional processing, motivational drive, executive functioning, pain, and memory (Bracht, Doidge, Keedwell, & Jones, Reference Bracht, Doidge, Keedwell and Jones2015; Bubb et al., Reference Bubb, Metzler-Baddeley and Aggleton2018). The functional specialization of distinct segments of the CB has been well-established. Specifically, the dorsal cingulum, which constitutes the uppermost component of the cingulum bundle and follows the trajectory of the cingulate gyrus, is predominantly associated with emotional regulation, social interactions, motivation, executive functioning, and pain (Bubb et al., Reference Bubb, Metzler-Baddeley and Aggleton2018; Budisavljevic et al., Reference Budisavljevic, Kawadler, Dell'Acqua, Rijsdijk, Kane, Picchioni and Catani2016). In contrast, the parahippocampal (PHP) cingulum, which is the posterior ventral part of the CB and runs within the PHP gyrus and retrosplenial cingulate gyrus, is more closely related to pain and memory (Bubb et al., Reference Bubb, Metzler-Baddeley and Aggleton2018).
The dorsal cingulum bundle (CB) is a vital structure that connects the PFC with the limbic system and plays a critical role in mood dysregulation (Bubb et al., Reference Bubb, Metzler-Baddeley and Aggleton2018). It contains fibers that form reciprocal connections between the PFC, ACC, and posterior cingulate cortex (PCC), which are involved in emotional regulation (Kobayashi & Amaral, Reference Kobayashi and Amaral2007; Vogt & Pandya, Reference Vogt and Pandya1987). The cingulum receives inputs from the cingulate cortex (Catani & De Schotten, Reference Catani and De Schotten2012), a brain area implicated in cognitive control of emotional processes affected by mood disorders (Bush, Luu, & Posner, Reference Bush, Luu and Posner2000; Versace et al., Reference Versace, Acuff, Bertocci, Bebko, Almeida, Perlman and Phillips2015). The dorsal cingulum fibers receive axons from the ACC (Catani, Howard, Pajevic, & Jones, Reference Catani, Howard, Pajevic and Jones2002), which regulates emotional processes. We know that the amygdala projections to frontal areas join the dorsal cingulum, in particular, efferents from the amygdala to the ACC join the dorsal cingulum (Heilbronner & Haber, Reference Heilbronner and Haber2014). It is important to note that existing literature indicates the involvement of the CB in the pathophysiology of MDD, further supporting its critical role in mood dysregulation (Bhatia et al., Reference Bhatia, Henderson, Hsu and Yim2018; Jin et al., Reference Jin, Delaparte, Chen, DeLorenzo, Perlman, Klein and Kotov2022). Although there have been studies examining CB microstructural changes in MDD samples, to our knowledge, few studies have specifically examined the relationship between CB microstructural changes, mood dysregulation, and NSSI in MDD adolescents with NSSI.
We aimed to investigate the white matter (WM) abnormalities in adolescents with depression, with and without NSSI, using diffusion tensor imaging (DTI). We utilized region of interest (ROI)-based tract-based spatial statistics (TBSS) and canonical ROI-based DTI analyses to examine DTI data on CB WM microstructure, focusing on fractional anisotropy (FA) as a measure of WM integrity and myelination (Curran, Emsell, & Leemans, Reference Curran, Emsell, Leemans, Van Hecke, Emsell and Sunaert2016; Friedrich et al., Reference Friedrich, Fraenz, Schlüter, Ocklenburg, Mädler, Güntürkün and Genç2020). We specifically investigated the cingulum bundle (CB), which is relevant to frontolimbic circuit dysfunction in MDD and plays a role in NSSI (Cohen, Elger, & Weber, Reference Cohen, Elger and Weber2008). Additionally, whole-brain analyses were conducted to identify potential WM changes in other tracts, including the uncinate and anterior corona radiate (Jiang et al., Reference Jiang, Zhao, Hu, Du, Chen, Wu and Gong2017; Westlund Schreiner et al., Reference Westlund Schreiner, Mueller, Klimes-Dougan, Begnel, Fiecas, Hill and Cullen2020). We hypothesized that adolescents with depression and NSSI (MDD/NSSI+) would exhibit lower mean FA values in the CB than those without NSSI (MDD/NSSI−) and healthy controls. We further speculated that higher NSSI severity and risk factors (DERS) would correlate with lower FA values. Given the impact of age and other factors on white matter development (Lebel & Beaulieu, Reference Lebel and Beaulieu2011), we conducted post hoc analyses on age, gender, illness duration, and depression severity. Finally, receiver-operating characteristics (ROC) were computed to assess the diagnostic sensitivity of differences in WM integrity between MDD/NSSI+ and MDD/NSSI− patients. Our findings contribute to understanding the neurobiological mechanisms of NSSI in adolescents with depression and may aid in the development of targeted interventions.
Methods
Participants
Adolescents with MDD between 12 and 21 years were consecutively recruited from inpatient units at the Clinic of Child and Adolescent Psychiatry, Affiliated Hangzhou First People's Hospital of Zhejiang University, China. Adolescents with acute psychotic symptoms, acute suicidality, pervasive developmental or psychotic disorders, unstable medical illnesses, or those with a contraindication to MRI (e.g. claustrophobic, mental implants, history of brain injury) were not included. Healthy participants who had never engaged in NSSI and who had neither received a psychiatric diagnosis in their lifetime nor undergone psychiatric treatment were recruited via public advertisement. The study was approved by the institutional ethics committee of Affiliated Hangzhou First People's Hospital of Zhejiang University and was performed following the Declaration of Helsinki. All subjects and their parents or guardians were informed about the study. Written consent was obtained from participants over 18, and from both participants and guardians for those under 18.
Clinical assessment
Following informed consent and assent (as appropriate), all participants completed comprehensive diagnostic assessments conducted by trained clinicians. Interviews were conducted separately with adolescents and parents. They included Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (K-SADS-PL) (Kaufman et al., Reference Kaufman, Birmaher, Brent, Rao, Flynn and Moreci1997) for participants under 18 years old and the Structured Clinical Interview for DSM-IV Axis I Disorders [SCID] (First, Reference First2002) 1for participants 18 years old or older. Depression severity was measured using the 24-item Hamilton rating scale for depression (HAMD) (Hamilton, Reference Hamilton1967).
We assessed NSSI severity using the Clinician-Rated Severity of Non-Suicidal Self-Injury (CRSNSSI) (APA, 2013). The CRSNSSI was used to assess the severity of non-suicidal self-injurious behaviors or problems as experienced by the individual in the past year. The clinician completes the measure on a 5-point scale (Level 0 = None; 1 = Subthreshold; 2 = Mild; 3 = Moderate; and 4 = Severe). Each level is described as follows: None (No NSSI acts or NSSI acts on fewer than three days and no urge to self-injure again.); Subthreshold (NSSI acts on 2–4 days or has self-injured in the past on five or more days and has reported urges to self-injure again.); Mild (NSSI acts on 5–7 days using a single method and not requiring surgical treatment.); Moderate (NSSI acts on 8–11 days using a single method and not requiring surgical treatment [other than cosmetic] or NSSI acts on 5–7 days using more than one method.); Severe (At least 1 NSSI act that required surgical treatment [other than cosmetic] or NSSI acts on 12 or more days using a single method or NSSI acts on eight or more days using more than one method.) (APA, 2013). If the participants' NSSI Severe level was more than Level 1, they were classified as MDD/NSSI+ group; if NSSI Severe level were Level 0, they were classified as MDD/NSSI− group.
The Difficulties with Emotion Regulation Scale (DERS) (Gratz & Roemer, Reference Gratz and Roemer2004), which include 36-item self-report items, was used to assess the following six emotion regulation difficulties: non-acceptance of emotions, difficulties engaging in goal-directed behavior, impulse control difficulties, lack of emotional awareness, self-perceived limited access to strategies, and lack of emotional clarity. All 36 items are scored along a five-point scale from 1 (Rarely) to 5 (Almost always). The DERS total score and six subscale scores were used as indicators of emotional regulation difficulties. Cronbach's alpha for the DERS in the present study was 0.957.
Image acquisition
MRI acquisition All participants underwent MRI scanning using a Siemens 3T MAGNETOM Verio scanner (Erlangen, Germany) with a 32-channel phased-array head coil. Diffusion-weighted images were acquired using pulsed gradient-spin-echo echo-planar-imaging (EPI) under the following parameters: repetition time (TR) = 11 700 ms; echo time (TE) = 86 ms; field of view (FOV) = 256 mm; acquisition matrix size = 128 × 128; slice thickness, 2 mm; b factor = 1000 s/mm2; flip angle = 90°; 65 axial slices and no slice gap; 30 direction diffusion; the total scanning time was approximately 11 min 50 s. In addition, a high-resolution 3D T1-weighted magnetization prepared rapid gradient-echo (MPRAGE) sequence was used with the following parameters: 1 mm isotropic voxels; slice thickness = 1 mm; 176 sagittal slices; acquisition matrix size = 256 × 256; TR = 1900 ms; TE = 2.52 ms; FOV = 256 mm; acquisition time, 4 min 18 s.
Image Data analyses
The DTI images were acquired using the PANDA software (Cui, Zhong, Xu, He, & Gong, Reference Cui, Zhong, Xu, He and Gong2013), which is a MATLAB toolbox that includes FSL, Diffusion Toolkit, and MRIcron. (http://www.nitrc.org/projects/panda/). The data were skull-stripped and corrected for eddy-current and head motion artifacts. The FA images were calculated on a voxel-by-voxel basis. The individual FA images were non-linearly registered to the Montreal Neurological Institute (MNI) space template using the FNIRT command of FSL. The mean FA image was generated by averaging all aligned FA images. An atlas-based segmentation approach was used to investigate diffusion changes in major white matter tracts. Specifically, each subject's FA map was registered to the JHU-ICBM WM Tractography Atlas (Hua et al., Reference Hua, Zhang, Wakana, Jiang, Li, Reich and Mori2008), and the FA values were extracted for the dorsal cingulum (left and right) and PHP cingulum (left and right) using the white matter probability map.
Statistical analysis for demographic, and clinical measures
We used SPSS 27.0 (SPSS, Inc., Chicago, Illinois) to analyze demographic and clinical characteristics. We compared demographics between healthy controls, patients with NSSI, and patients without NSSI using ANOVA, two-sample t tests, or χ2 tests as appropriate for continuous or dichotomous variables.
Whole-brain and ROI-restricted TBSS analyses
The present study aimed to investigate group differences in FA using whole-brain and ROI-restricted TBSS analyses. To this end, a series of standard voxelwise analyses were performed in FSL, following established procedures (Smith et al., Reference Smith, Jenkinson, Johansen-Berg, Rueckert, Nichols, Mackay and Behrens2006). First, the FMRIB58 FA 1 mm standard space template image was used as the registration target for each subject's FA image, which was aligned into standard 1 × 1 × 1 mm MNI152 space using the nonlinear registration tool FNIRT. The individual subjects' FA images were then merged into a single 4D image, and the mean FA volume was calculated and thinned to generate the mean FA skeleton. The mean FA skeleton was thresholded at 0.2 to exclude peripheral tracts with low FA values. Then, individual subjects' aligned FA images were projected onto this skeleton, and the resulting skeletonized FA data were fed into the voxelwise nonparametric permutation test using randomize, a nonparametric permutation-based inference tool for thresholding statistical maps (Winkler, Ridgway, Webster, Smith, & Nichols, Reference Winkler, Ridgway, Webster, Smith and Nichols2014).
For the ROI-restricted TBSS analysis, masks were created by multiplying the mean FA skeleton mask, which was binarized and thresholded at 0.95 (i.e. p = 0.05), with each of the four regions of interest (ROIs) using fslmaths (Hubbard et al., Reference Hubbard, Becerra, Heinz, Ludwick, Rasooly, Yendiki and Borsook2018). Specifically, the ROIs corresponded to the cingulum bundles, which were obtained from the ICBM-DTI-81 1 mm FA atlas (Mori et al., Reference Mori, Oishi, Jiang, Jiang, Li, Akhter and Mazziotta2008) of the Johns Hopkins University (JHU) DTI-based white-matter tractography atlas in FSL. The dorsal cingulum bundle consisted of the right and left subgenual and retrosplenial subdivisions combined, while the ventral cingulum bundle included the right and left ventral aspects.
Voxel-wise statistics on skeletonized FA were conducted between groups using two-sample t tests, with age and gender specified as covariates of no interest and each of the four masks flagged using randomize. The resulting significant TFCE-corrected p value statistical maps were thresholded at 0.95 (i.e. p = 0.05) and binarized to create masks used to extract mean FA values from significant clusters using FSL meants command with the 4D skeletonized FA image specified as the input. Extracted mean FA values were imported to SPSS (version 27), and violin graphs were created to visualize differences in group means.
In addition to the aforementioned structures, we also investigated the uncinate fasciculus (UF), superior longitudinal fasciculus (SLF), and the Anterior Thalamic Radiation to comprehensively test the hypothesis of impaired connectivity between frontal areas and the limbic system.
Canonical ROI-based DTI Analysis
We conducted a canonical ROI-based DTI analysis using PANDA. We registered all subjects' native T1 images to the MNI T1 1 mm brain template and nonlinearly registered their native FA images to the MNI FMRIB58 FA 1 mm template. We deprojected the four ROIs (R and L dorsal cingulum bundle and ventral cingulum bundle) from the JHU ICBM-DTI-81 1 mm FA atlas and extracted mean FA values. We imported the extracted values from the four ROIs for each subject into SPSS and used mixed-model MANCOVA, controlling for age, gender, and depression severity, to compare the between-subject factor subgroups (MDD/NSSI+, MDD/NSSI−, and healthy controls) for further analysis. All p values were corrected for multiple comparisons using Bonferroni adjustment. A two-tailed significance level of p < 0.05 was used for all analyses.
Spearman correlations were calculated between Clinical indicators and FA of CB for each fraction. To account for the effects of gender, adolescent development, and depression severity on white matter, we controlled the effects of gender, age, HAMD scores, and Illness duration (years).
We used ROC analyses to evaluate the ability of mean FA values from CB to discriminate between MDD/NSSI+ and MDD/NSSI− patients. To control for age-related changes in white matter, we included age as a covariate and mean FA values from significant CB regions as independent variables in a binary logistic regression model, with the diagnostic group as the dependent variable. The predicted probability output from the model was used as the test variable, and the diagnostic group was used as the state variable, assuming a nonparametric distribution. The cutoff scores that yielded the best balance between sensitivity and specificity (i.e. Youden Index) were used to calculate sample-based sensitivity/specificity proportions. Additionally, we incorporated the DERS total score and the mean values of FA from CB regions of significant effect using the same method to enhance the classification performance between groups.
Results
Demographics
Demographic and clinical characteristics are shown in Table 1. A total of 130 individuals completed the study, including 35 healthy controls (HC group), 48 MDD patients without NSSI (MDD/NSSI- group), and 47 patients with NSSI (MDD/NSSI+ group), completed the study. There were no significant differences in age or education among the three groups. However, the MDD/NSSI+ group had a significantly higher proportion of females than the MDD/NSSI− group. The MDD/NSSI+ patients demonstrated the highest scores on the HAMD and DERS total and subscale scores, followed by the MDD/NSSI− group and the HC group. Analysis of variance (ANOVA) revealed a linear trend in the scores. Finally, there were no significant differences in medication status (psychotropic medication intake) or illness duration between MDD/NSSI+ and MDD/NSSI− patients.
Table 1. Characteristics of the sample

Note. The data are shown as mean (s.d.) or number.
s.d., standard deviation; N, sample size; HC, healthy controls; MDD/NSSI−, patients without non-suicidal self-injury group; MDD/NSSI+, patients with non-suicidal self-injury; HAMD, Hamilton Depression Rating Scale; DERS, Difficulties with Emotion Regulation Scale; CRSNSSI, Clinician-Rated Severity of Non-Suicidal Self-Injury; Medication, antidepressants, antipsychotics, mood stabilizers.
Group difference of FA values in the cingulum bundle
The current study employed both ROI-restricted TBSS and canonical ROI-based DTI analysis approaches to examine differences in mean FA of the dorsal and PHP cingulum bundles among the three groups. The results of the ROI-restricted TBSS indicated a significant group difference in mean FA for the left dorsal cingulum (p < 0.05, TFCE and FWE corrected) between the MDD/NSSI + and HC groups. In contrast, no significant difference was found for the right dorsal cingulum (see Fig. 1a). Furthermore, a significant group difference in mean FA for both the right and left dorsal cingulum (p < 0.05, TFCE and FWE corrected) was observed between the MDD/NSSI+ and MDD/NSSI− groups (see Fig. 1b). However, no group difference in mean FA was found for the PHP cingulum. The whole-brain TBSS analysis also showed no significant group differences in WM mean FA, except for the cingulum bundle, which exhibited a significant difference (p < 0.05, TFCE and FWE corrected) between the MDD/NSSI+ group, MDD/NSSI− group, and HC group.

Figure 1. Results from the ROI-restricted TBSS group analysis for the dorsal cingulum.
Note. a, ROI-restricted TBSS analysis revealed a noteworthy reduction in FA observed in the dorsal cingulum cluster of MDD/NSSI+ in comparison to HC (MDD/NSSI+ < HC; p < 0.05, TFCE and FWE corrected; the color bar is scaled from 0.95 to 1), with the peak voxel in the FA cluster being located at MNI coordinates x = −9.2, y = 8.8, z = 23.8. b, ROI-restricted TBSS analysis revealed a noteworthy reduction in FA in MDD/NSSI+ in contrast to MDD/NSS− (MDD/NSSI+ < MDD/NSS−; p < 0.05, TFCE and FWE corrected; color bar is scaled from 0.95 to 1) for a cluster located in the dorsal cingulum, with the peak voxel in the FA cluster being located at MNI coordinates x = −10.1, y = 3.5, z = 33.6. c, cluster graph displaying the mean values extracted from a significant FA cluster in the left dorsal cingulum bundle among three groups using canonical ROI-based DTI analysis. d. cluster graph displaying the mean values extracted from the significant FA cluster in the right dorsal cingulum bundle across the three groups, using canonical ROI-based DTI analysis, and an asterisk is used to indicate the significant differences between groups. FA, fractional anisotropy; CB, cingulum bundle; HC, healthy controls; MDD/NSSI−, patients without non-suicidal self-injury group; MDD/NSSI+, patients with non-suicidal self-injury; PHP, parahippocampal; Asterisk denotes significant group differences; TFCE, Threshold Free Cluster Enhancement; FEW, Family-Wise Error.
By using canonical ROI-based DTI analysis, we found that MANCOVAs revealed a main effect of group (F (2,127) = 6.151, p = 0.003), as well as a significant effect of hemisphere (F (1,127) = 412.477, p < 0.001), but no significant group × hemisphere interaction (F (2,127) = 0.006, p = 0.994) for FA in the dorsal cingulum bundle. Consistent with the ROI-restricted TBSS analysis, controlling for age, gender, illness duration, and HAMD scores showed significant group effects for both the left and right dorsal cingulum (F (2, 89) = 5.541, p = 0.005; F (2, 89) = 7.213, p = 0.001, respectively). The reduction in FA observed in the MDD/NSSI+ subgroup was the main driver of these differences, compared to the MDD/NSSI- and HC subgroups (see Table 2 and Fig. 1c and 1d). Furthermore, a main effect of group was found for FA in the right PHP cingulum (F (2, 89) = 3.769, p = 0.027), but not in the left PHP cingulum (F (2, 89) = 2.961, p = 0.057).
Table 2. ANCOVAs controlling for age, gender, illness duration, and depression severity comparing mean FA of CB between MDD/NSSI+, MDD/NSSI− and healthy controls subgroups
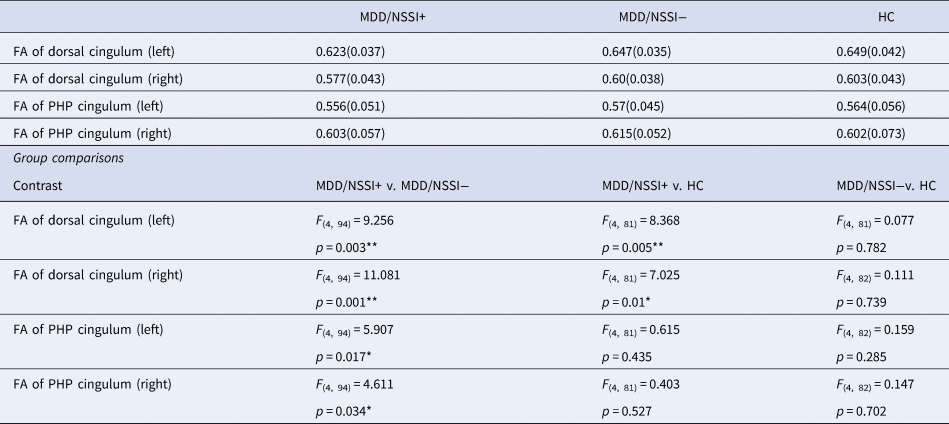
Note. FA, fractional anisotropy; CB, cingulum bundle; HC, healthy controls; MDD/NSSI−, patients without non-suicidal self-injury group; MDD/NSSI+, patients with non-suicidal self-injury; PHP, parahippocampal; Asterisk denotes significant group differences.
Contrary to the ROI-restricted TBSS analysis, which did not yield positive results, the canonical ROI-based DTI analysis revealed significant group effects for the right SLF (F (2, 130) = 3.224, p = 0.043) when controlling for age, gender, and HAMD scores. However, no group differences were observed in other white matter fibers (See online Supplementary Material, Tables S5 and S6).
Association analyses between cingulum and clinical characteristics related to NSSI
After adjusting for potential confounding variables, including age, gender, illness duration, and HAMD scores, a significant negative correlation was observed between the mean FA of the dorsal cingulum and the severity of NSSI (r = −0.344, p = 0.005).
In the group of MDD/NSSI+, no correlation was observed between any segments of the cingulum and the total score or subscale scores of DERS. However, a notable inverse relationship was identified between the left dorsal cingulum and the DERS total score among all participants, regardless of covariate adjustment (r = −0.263, p = 0.003; r = −0.209, p = 0.019, Covariate: HAMD scores; r = −0.185, p = 0.039, Covariates: age, gender, illness duration, and HAMD scores). (See online Supplement Tables S1–S3, Fig. 2). Incorporation of HAMD scores as a covariate in the analysis revealed that solely the DERS total score, Impulse, and Strategies subscale scores demonstrated a significant association with the mean FA of the left dorsal cingulum (see Supplement Table S2). Additionally, a negative correlation was observed between the DERS total score, Impulse, Strategies, and Clarity subscale scores, and the FA of the right PHP cingulum (see online Supplement Table S2). Upon adjustment for nuisance covariates, including age, gender, illness duration, and HAMD scores, solely the DERS total score and Clarity score were negatively associated with the mean FA in the right PHP cingulum (see online Supplement Table S3).
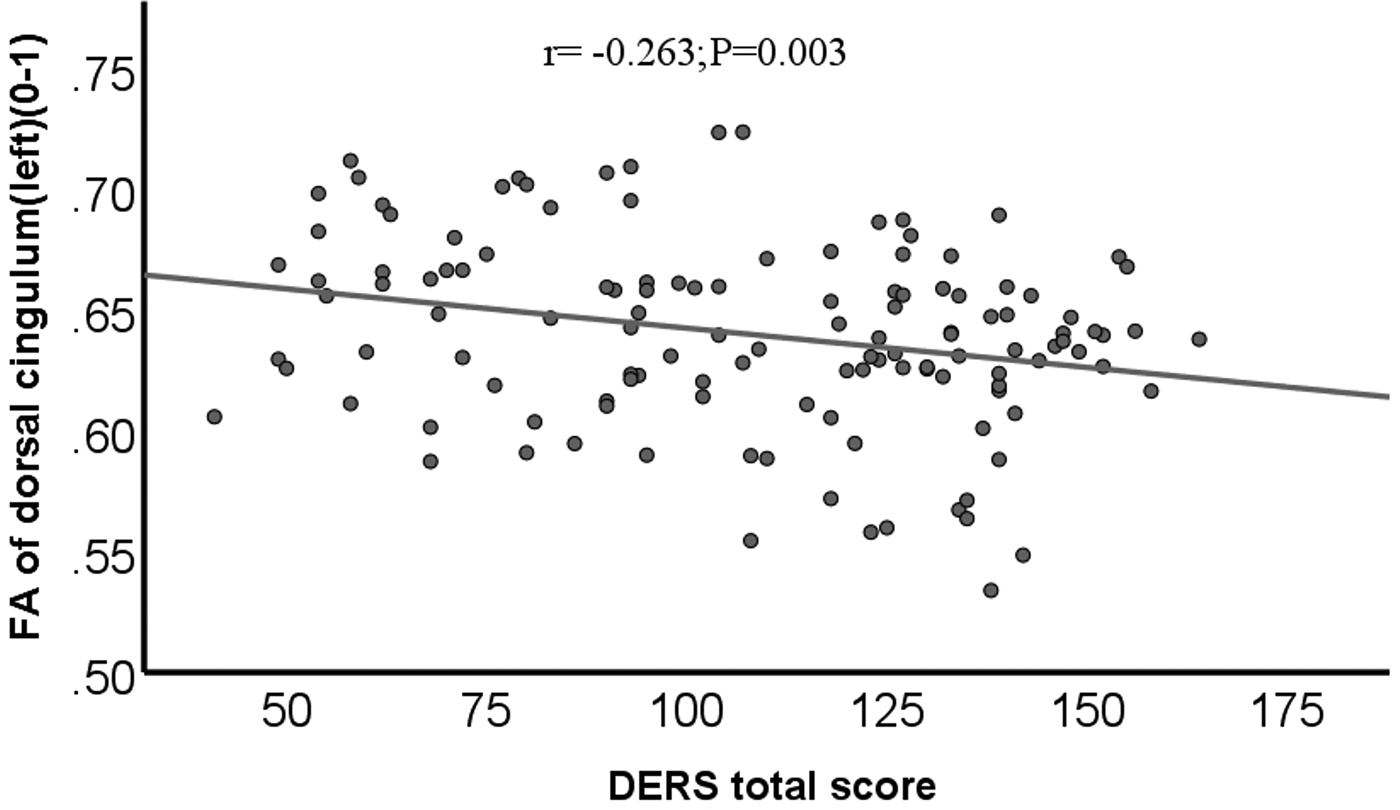
Figure 2. Scatter plot of FA in left dorsal cingulum negatively associated with the DERS total score.
Note. FA, fractional anisotropy; DERS, Difficulties with Emotion Regulation Scale.
Receiver-operating characteristics (ROC)
The DERS scores demonstrated moderate discriminatory ability in distinguishing between MDD/NSSI+ and MDD/NSSI−, as evidenced by an area under the curve (AUC) of 0.70 (s.e. = 0.05, p = 0.001, CI 0.60–0.80) (online Supplement Table S4). The microstructural integrity of the CB region, as measured by mean FA, also demonstrated moderate discriminatory ability in distinguishing between MDD/NSSI+ and MDD/NSSI− patients, with an AUC of 0.71 (s.e. = 0.05, p = 0.001, CI 0.60–0.81), a sensitivity of 74.5%, and a specificity of 56.2% (see Fig. 3). By incorporating both CB microstructural integrity and DERS scores, there was relatively successful discrimination between MDD/NSSI + and MDD/NSSI- patients, with an AUC of 0.81 (s.e. = 0.04, p < 0.001, CI 0.73–0.90), a sensitivity of 70%, and a specificity of 83% (see Fig. 3).
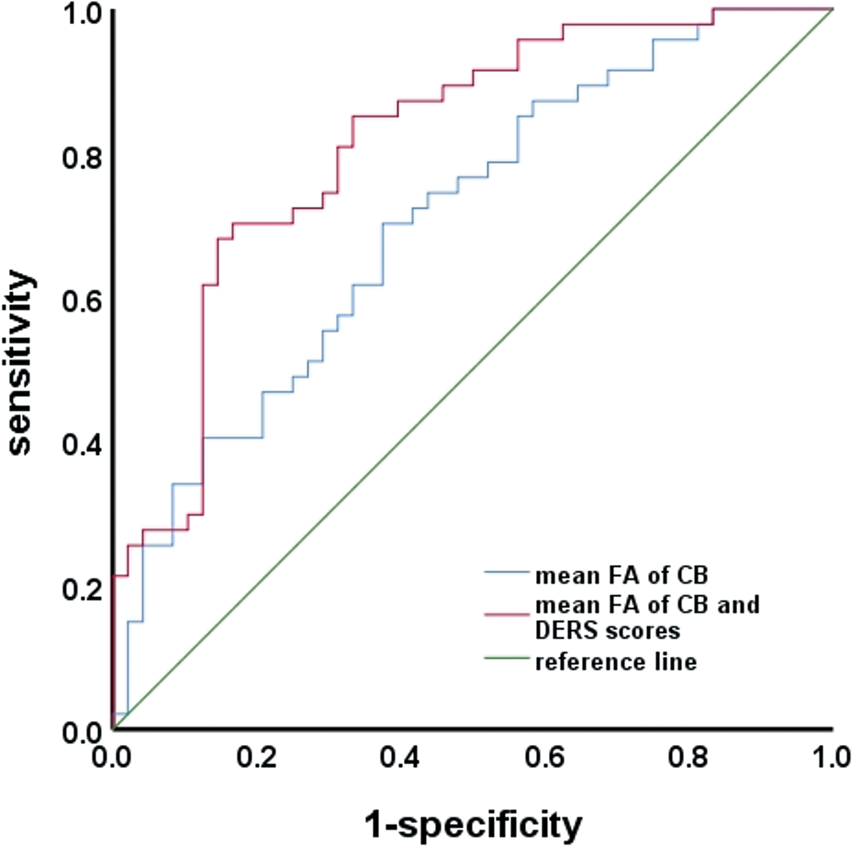
Figure 3. Receiver-operating characteristics (ROC) analysis.
Note. FA, fractional anisotropy; DERS, Difficulties with Emotion Regulation Scale; CB, cingulum bundle.
Discussion
The study found evidence of white matter microstructural abnormalities in the left dorsal cingulum of adolescents with MDD and NSSI, which were linked to increased severity of NSSI, independent of clinical and demographic factors. The integration of cingulum microstructural integrity and DERS scores allowed for successful differentiation between MDD/NSSI+ and MDD/NSSI− patients. The present findings underscore the significance of the dorsal cingulum in the etiology of NSSI and contribute to a deeper understanding of NSSI through the imbalance theory of frontolimbic circuits (Kaess et al., Reference Kaess, Hooley, Klimes-Dougan, Koenig, Plener, Reichl and Cullen2021;Santamarina-Perez et al., Reference Santamarina-Perez, Romero, Mendez, Leslie, Packer, Sugranyes and Singh2019). Our findings add to the current body of literature on white matter abnormalities in NSSI, confirming and expanding on previous research (Auerbach et al., Reference Auerbach, Pagliaccio, Allison, Alqueza and Alonso2021;Westlund Schreiner et al., Reference Westlund Schreiner, Mueller, Klimes-Dougan, Begnel, Fiecas, Hill and Cullen2020).
Our study findings reveal that adolescents in the MDD/NSSI+ group exhibit reduced FA in the dorsal CB, which is consistent with the previous work of Schreiner et al. (Westlund Schreiner et al. Reference Westlund Schreiner, Mueller, Klimes-Dougan, Begnel, Fiecas, Hill and Cullen2020). However, they identified a broader range of white matter modifications, including the uncinate, upper longitudinal band, and other changes, in addition to the CB. This may be due to our control for psychopathology diagnosis, while their study was cross-diagnostic. Notably, Schreiner et al., acknowledged the possible impact of psychopathology diagnosis on their findings (Westlund Schreiner et al., Reference Westlund Schreiner, Mueller, Klimes-Dougan, Begnel, Fiecas, Hill and Cullen2020). Our research enhances the current literature by exploring different CB compartments and their ability to differentiate between MDD with and without NSSI. Furthermore, our analysis revealed a significant correlation between dorsal CB microstructure and NSSI. Importantly, no differences in CB microstructure were found between the MDD/NSSI− and healthy control groups, implying that the alterations observed in the dorsal CB are specifically linked to NSSI rather than depression.
Following adjustments for potential confounding variables, partial correlation analyses demonstrate a significant inverse correlation between the severity of NSSI and the mean FA of the dorsal CB. Given that the MDD/NSSI+ subtype represents a distinct clinical subgroup with unique behavior patterns, our finding of reduced FA in the dorsal CB may serve as a potential biological marker of NSSI. However, it should be noted that longitudinal assessments would be necessary to establish the causal relationship between dorsal CB microstructure and NSSI. Although the findings presented in this study underscore the significance of the dorsal CB in the context of NSSI, it is necessary to conduct additional research to explore and validate this association further, as the modulation of human emotions by frontolimbic circuits is a complex process that requires further investigation.
Contrary to our expectations, we did not find a correlation between mean FA within the CB and DERS scores in the NSSI group. However, we did observe a negative correlation between the mean FA of the dorsal cingulum and the severity of DER among all participants. This suggests that reduced FA in the left dorsal CB may be associated with increased severity of DER, consistent with previous research (Versace et al., Reference Versace, Acuff, Bertocci, Bebko, Almeida, Perlman and Phillips2015). It is important to note that our results were only observed among all participants and not specifically within the MDD/NSSI+ or MDD groups. Given the heterogeneity between groups, we cannot solely rely on the theory of DER to explain the association between CB and NSSI. Although existing theoretical models and empirical investigations converge on the notion that NSSI is a manifestation of DER (Hu et al., Reference Hu, Huang, Shang, Huang, Jiang and Yuan2023;Yurkowski et al., Reference Yurkowski, Martin, Levesque, Bureau, Lafontaine and Cloutier2015), further research is necessary to elaborate on these relationships by incorporating measures that investigate specific DER features of NSSI in adolescents with depression.
Our study revealed specific alterations in WM within the dorsal region of the CB. In addition, the clarity subscale score was linked to the right PHP region, suggesting that emotional clarity involves the hippocampus and PCC (Jones, Christiansen, Chapman, & Aggleton, Reference Jones, Christiansen, Chapman and Aggleton2013). We also observed that WM changes in the CB were restricted to the left hemisphere, consistent with Schreiner's previous findings (Westlund Schreiner et al., Reference Westlund Schreiner, Mueller, Klimes-Dougan, Begnel, Fiecas, Hill and Cullen2020). However, it is worth noting that an interhemispheric imbalance is frequently observed in depressive disorders, where lesions in the left hemisphere are known to induce depressed mood (Aghajani et al., Reference Aghajani, Veer, Van Lang, Meens, Van den Bulk, Rombouts and Van der Wee2014). Our results thus support the idea that interhemispheric imbalance could contribute to the pathophysiology of depression with NSSI.
The study's strengths include the inclusion of the MDD/NSSI− group as a control to manage confounding effects of other psychopathologies, the use of CRSNSSI to evaluate NSSI, improving the homogeneity of self-harm, and the application of whole-brain and ROI analyses using TBSS to minimize registration errors and partial volume, providing a more robust and sensitive assessment of white matter microstructure. However, the present study acknowledges several limitations that could impact the interpretation of its findings. Firstly, age significantly impacts FA during adolescence (Lebel & Beaulieu, Reference Lebel and Beaulieu2011). However, after adjusting for age, our model maintained comparable discriminatory power (see online Supplement Table S4). Still, a future multi-measure approach is needed. Secondly, the cross-sectional data precludes drawing causal inferences. Thirdly, the small number of male participants in the MDD/NSSI+ group limits the generalizability of the findings. Lastly, it is essential to note that some participants were medicated, which could have influenced the results. To eliminate the potential impact of medication, future studies should consider validating the findings in a non-medicated patient cohort.
Conclusion
Our research reveals that adolescents with MDD who engage in NSSI exhibit abnormal white matter microstructure in the left dorsal cingulum. This abnormality is associated with the severity of NSSI independent of clinical and demographic factors. These findings highlight the importance of the dorsal cingulum in developing NSSI and support a frontolimbic theory related to emotion regulation function in adolescents. The combination of cingulum integrity and DERS scores could enable the identification of MDD patients with NSSI and serve as an early-stage diagnostic tool. However, this needs to be validated by future longitudinal studies in representative samples with a larger size. Future research should aim to conduct longitudinal studies with DTI investigations of individuals at risk for NSSI, examining cingulum microstructure measures to determine whether these alterations are state or trait markers.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003329172300291X
Acknowledgments
We thank all the subjects for participating in this study. We also thank Shihui Huang for assistance with MRI data collection.
Funding statement
This work is supported by the Zhejiang Medical Association Program (grant number: 2023KY920) and the National Natural Science Foundation of China (grant number: 81971277). The funders peer-reviewed this study protocol before granting the funding. The funders had no role in operation of this project and did not have any involvement in the preparation, review, approval, or decision to submit this manuscript for publication.
Competing interest
None.
Ethical standards
The study was conducted by the Declaration of Helsinki and approved by the ethics committee of the Affiliated Hangzhou First People's Hospital of Zhejiang University (IRB: 2020-K008–01, January 2020). The patients or their guardians provided written informed consent to participate in this study.








