It has been estimated that the prevalence of type 2 diabetes in people with schizophrenia is around 2-4 times higher than in the general population (Reference Bushe and HoltBushe & Holt, 2004). The complications of type 2 diabetes can lead to significant physical morbidity but these may be limited by effective monitoring and good early management of the disease (National Institute for Clinical Excellence, 2002a ).
Evidence suggests that treatment with antipsychotic medication increases the risk of developing diabetes. Although most studies are retrospective they generally report a higher rate of diabetes in those on atypical preparations compared with typical antipsychotics (Reference HaddadHaddad, 2004). It remains uncertain whether any particular atypical preparation is more likely to cause type 2 diabetes than another and it is therefore prudent to consider the changes in glucose metabolism as a class effect for these drugs (Reference Koro, Fedder and L'ItalienKoro et al, 2002; Reference Newcomer, Haupt and FucetolaNewcomer et al, 2002; Reference Howes, Bhatnagar and GaughranHowes et al, 2004).
Since a diagnosis of schizophrenia and its treatment with an antipsychotic both increase the risk of impaired glucose tolerance and diabetes, patients should expect routine, standardised monitoring and screening (Reference Haupt and NewcomerHaupt & Newcomer, 2001; Reference Boilson and HamiltonBoilson & Hamilton, 2003; Expert Group, 2004). In-patient surveys of blood glucose monitoring and testing for diabetes in those on antipsychotics indicate that this is neither routine nor systematic and little is known about the screening practices in primary care/community populations prescribed these medications (Reference Boilson and HamiltonBoilson & Hamilton, 2003; Reference Taylor, Young and EsopTaylor et al, 2004).
Method
The sample comprised all adult patients in a sector of rural Derbyshire who were prescribed atypical antipsychotics in 2004. Standards were developed based on the proposed pragmatic pathway for managing diabetes risks in those prescribed atypical antipsychotics from the consensus summary of the Expert Group (2004), the Maudsley Prescribing Guidelines (Reference Taylor, Patton and KerwinTaylor et al, 2003), the National Institute for Clinical Excellence (NICE) guidelines (NICE, 2002b ), clinicians' best practice and local service expectations. The standards are shown in Table 1.
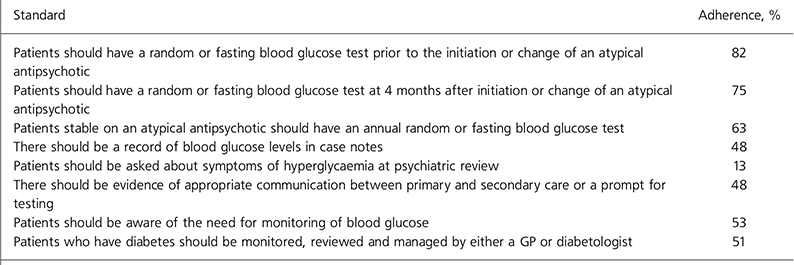
Table 1. Adherence to audit standards
| Standard | Adherence, % |
|---|---|
| Patients should have a random or fasting blood glucose test prior to the initiation or change of an atypical antipsychotic | 82 |
| Patients should have a random or fasting blood glucose test at 4 months after initiation or change of an atypical antipsychotic | 75 |
| Patients stable on an atypical antipsychotic should have an annual random or fasting blood glucose test | 63 |
| There should be a record of blood glucose levels in case notes | 48 |
| Patients should be asked about symptoms of hyperglycaemia at psychiatric review | 13 |
| There should be evidence of appropriate communication between primary and secondary care or a prompt for testing | 48 |
| Patients should be aware of the need for monitoring of blood glucose | 53 |
| Patients who have diabetes should be monitored, reviewed and managed by either a GP or diabetologist | 51 |
Patients were identified using psychiatric case notes, trust pharmacy records and general practitioner (GP) prescribing records. Information was gathered from multiple sources, including pathology and pharmacy records and psychiatric and GP case notes. Blood monitoring took place at Derby City Hospital. All information was recorded using an audit sheet designed to investigate the standards. A pilot study of five patients was initially undertaken. Diagnoses were recorded according to ICD-10 (World Health Organization, 1993) from the trust register and using information in medical notes. Basic descriptive statistics have been used to collate results.
Results
Population, diagnosis and prescriptions
The sector covers a total population of approximately 40 000. There were a total of 55 patients on atypical antipsychotic medications (30 men and 25 women); 41 had a primary diagnosis of functional psychosis (ICD-10 codes F20-29), 12 an affective psychosis (F30-39) and 2 other diagnoses of organic mania (F06.3) and emotionally unstable personality (F60.3) respectively. The audit was completed on the total number of atypical prescription scripts (60) over the year January to December 2004. This accounted for some patients who within the year had both started and changed atypical antipsychotic medication.
There were 10 patients who had a definitive diagnosis of type 2 diabetes. Their average age at the time of audit was 53 years and their median duration of treatment 40 months. There were 7 that had been diagnosed with diabetes after prescription of an atypical antipsychotic; 3 had been diagnosed within the year of the audit by their GP following a routine random blood glucose and subsequent glucose tolerance test.
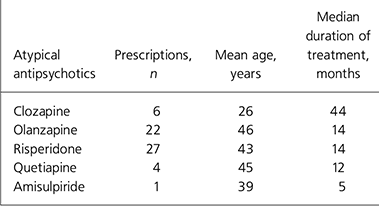
Table 2 gives details of the drugs prescribed, the mean age of the patients and the median duration of treatment for each medication.
Table 2. Atypical antipsychotics according to number of prescriptions, patient age and duration of treatment
| Atypical antipsychotics | Prescriptions, n | Mean age, years | Median duration of treatment, months |
|---|---|---|---|
| Clozapine | 6 | 26 | 44 |
| Olanzapine | 22 | 46 | 14 |
| Risperidone | 27 | 43 | 14 |
| Quetiapine | 4 | 45 | 12 |
| Amisulpiride | 1 | 39 | 5 |
Standards
Adherence to each of the standards is shown in Table 1. The overall number of instances of initiation or change of atypical antipsychotics was 24. In 13 instances this occurred during an in-patient stay with a random or fasting blood glucose test in accordance with the first standard. In 11 instances the initiation or change took place in an out-patient setting; in only 2 was a blood glucose test recorded. Fifteen of those initiated or changed also reached 4 months' duration of treatment within the audit year. All of those who were in-patients at 4 months had a random blood glucose test. None of those reaching 4 months' duration of treatment as out-patients had such a test.
There was a noticeable difference in the rates of testing for different medications. Those stable on olanzapine and clozapine were more likely to have had tests performed.
All patients who were known to have diabetes were receiving appropriate care for this. However, adherence to the last standard was only 51%, as patients who had not had a blood test and had missing data were considered potentially to have diabetes.
Discussion
This medical audit compared our practices with emerging, growing and more definitive evidence about the longer-term side-effects of antipsychotic treatment. It has been conducted with an awakening awareness of the lifestyle and physical health needs of those with severe and enduring mental illnesses (Reference Connolly and KellyConnolly & Kelly, 2005). Differences in the surveillance practice for each atypical antipsychotic are in accordance with previous research (Reference Citrome, Jaffe and LevineCitrome et al, 2004; Reference Taylor, Young and EsopTaylor et al, 2004). This might reflect the current lack of a clear consensus from multiple and differing guidelines on the necessary monitoring for both primary and secondary care. It is noteworthy that the most common reference used for prescribing, the British National Formulary (http://bnf.org/bnf), suggests that olanzapine and clozapine are more liable to cause hyperglycaemia or diabetes as side-effects.
There were large differences between the routine monitoring of blood glucose in in-patient and out-patient settings. In-patients benefited from a physical examination with routine physical tests at the time of admission to the ward. However, a random blood glucose test was not necessarily a standard and routine minimum requested by the admitting doctor. This concurs with the findings of Boilson & Hamilton (Reference Boilson and Hamilton2003) that for psychiatric admission physical tests are often performed on an ad hoc basis.
Blood glucose testing for out-patients was markedly different and poor. The main barrier to both the initiation and routine monitoring of blood glucose as an out-patient was effective communication between services and consistency of information. The standard form of communication between the psychiatrist and GP remains a letter and/or telephone conversation. Requests for testing of out-patients in letters to the GP may be and were easily missed. There have been no previous studies investigating the physical monitoring of psychiatric out-patients. However, the need for improved communication and coordination of services between primary and secondary care is well recognised and emphasised by this audit.
Any assumption by psychiatrists that all physical care is the territory of the GP can hinder the monitoring of psychiatric treatment. The roles and responsibilities of primary and secondary care can seem unclear. Emphasis has been placed on the importance of psychiatrists arranging appropriate monitoring of medications that they are prescribing. Furthermore, advice and information given to the patient about their treatment is essential for good clinical practice (NICE, 2002b ) and aids adherence to treatment and monitoring. Psychiatrists therefore need to work closely with GPs and their patients to ensure this happens (Reference Connolly and KellyConnolly & Kelly, 2005).
There is a new emphasis on the need to attend to the long-overlooked physical health of those with severe and enduring mental illness. The new GP contract, the development of registers in primary care, guidance on clinical indicators (British Medical Association, 2004) and the care programme approach have already given impetus for consistency and routine monitoring in these areas. Opportunities exist to take advantage of this locally by developing a standard and coordinated approach across primary care and mental health trusts. With some limited and preliminary evidence that lifestyle changes can reduce the random blood glucose level in those on clozapine (Reference Smith and WhiteSmith & White, 2004), there could also be potential practical preventive interventions for patients on these treatments.
Declaration of interest
None.





eLetters
No eLetters have been published for this article.