Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is estimated to have caused more than 18 million deaths worldwide until the end of 2021(1). Early in the pandemic, the striking overlap between risk factors for severe disease and those for vitamin D deficiency – older age, obesity and South Asian or Black ethnic origin – gave rise to speculation that vitamin D supplementation might have a role in the prevention or treatment of COVID-19(Reference Martineau and Forouhi2,Reference Bhala, Curry and Martineau3) . This hypothesis was supported by mechanistic data relating to favourable immunomodulatory actions of vitamin D in the context of other viral respiratory infections(Reference Greiller, Suri and Jolliffe4–Reference Jolliffe, Greiller and Mein6), and by findings from meta-analyses of data from randomised controlled trials (RCTs), which demonstrated protective effects of vitamin D supplementation against acute respiratory infections caused by pathogens other than SARS-CoV-2(Reference Martineau, Jolliffe and Hooper7,Reference Jolliffe, Camargo and Sluyter8) .
Since that time, a large body of evidence from laboratory studies, epidemiological investigations, RCTs and meta-analyses has accumulated in this field. This review describes the mechanisms by which hydroxylated metabolites of vitamin D may modulate host responses to SARS-CoV-2 infection and vaccination, before going on to discuss findings of observational and intervention studies that have been conducted to establish whether vitamin D supplementation is clinically indicated (1) for the treatment of COVID-19; (2) for the prevention of COVID-19 and/or (3) as an adjunct to vaccination against SARS-CoV-2 (Fig. 1).

Fig. 1. Potential clinical applications of vitamin D supplementation for coronavirus disease 2019 (COVID-19).
Mechanism of action
Vitamin D is a group of fat-soluble vitamins: vitamin D3 (cholecalciferol) is the major form in human subjects: its primary source is via cutaneous synthesis in response to sunlight, but it can also be ingested orally, from dietary sources (such as oily fish) or in supplements(Reference Holick9). Fig. 2 illustrates mechanisms by which vitamin D may modulate host immune responses to SARS-CoV-2 infection. Vitamin D from cutaneous synthesis or oral intake is converted to 25-hydroxyvitamin D [25(OH)D, the major circulating metabolite and measure of vitamin D status], primarily by the liver. Respiratory viruses ligate pattern recognition receptors to induce expression of the 25(OH)D hydroxylase CYP27B1 in pulmonary epithelium and leucocytes. This enzyme catalyses conversion of 25(OH)D to its active vitamin D metabolite 1,25-dihydroxyvitamin D [1,25(OH)2D]. 1,25(OH)2D3 ligates the vitamin D receptor to regulate gene expression profiles, resulting in upregulation of antiviral effector mechanisms (expression of antimicrobial peptides including cathelicidin LL-37 and human β defensin 2, interferon-stimulated genes and generation of reactive oxygen and nitrogen intermediates) with potential to reduce susceptibility to infection and severity of disease. It also regulates inflammation by modulating innate immune responses (regulating NF-κB and mitogen-activated protein kinase pathways to reduce expression and secretion of pro-inflammatory cytokines and increasing the ratio of angiotensin converting enzyme 2 [ACE2] to ACE)(Reference Malek Mahdavi10) and by regulating adaptive responses to inhibit differentiation of null T helper cells towards type 1 or type 17 phenotypes and to promote their differentiation towards a T regulatory phenotype(Reference Bikle11). In the context of active COVID-19, these anti-inflammatory actions have potential to reduce disease severity associated with ‘cytokine storms’(Reference Ragab, Salah Eldin and Taeimah12). In the context of vaccination, they may serve to augment development of antigen-specific immunity(Reference Chambers, Vukmanovic-Stejic and Turner13). Finally, 1,25(OH)2D may also support classical T cell receptor signalling and T cell activation by inducing phospholipase C-gamma 1 in naïve T cells(Reference von Essen, Kongsbak and Schjerling14). These actions would also be expected to support development of antigen-specific immunity following vaccination.
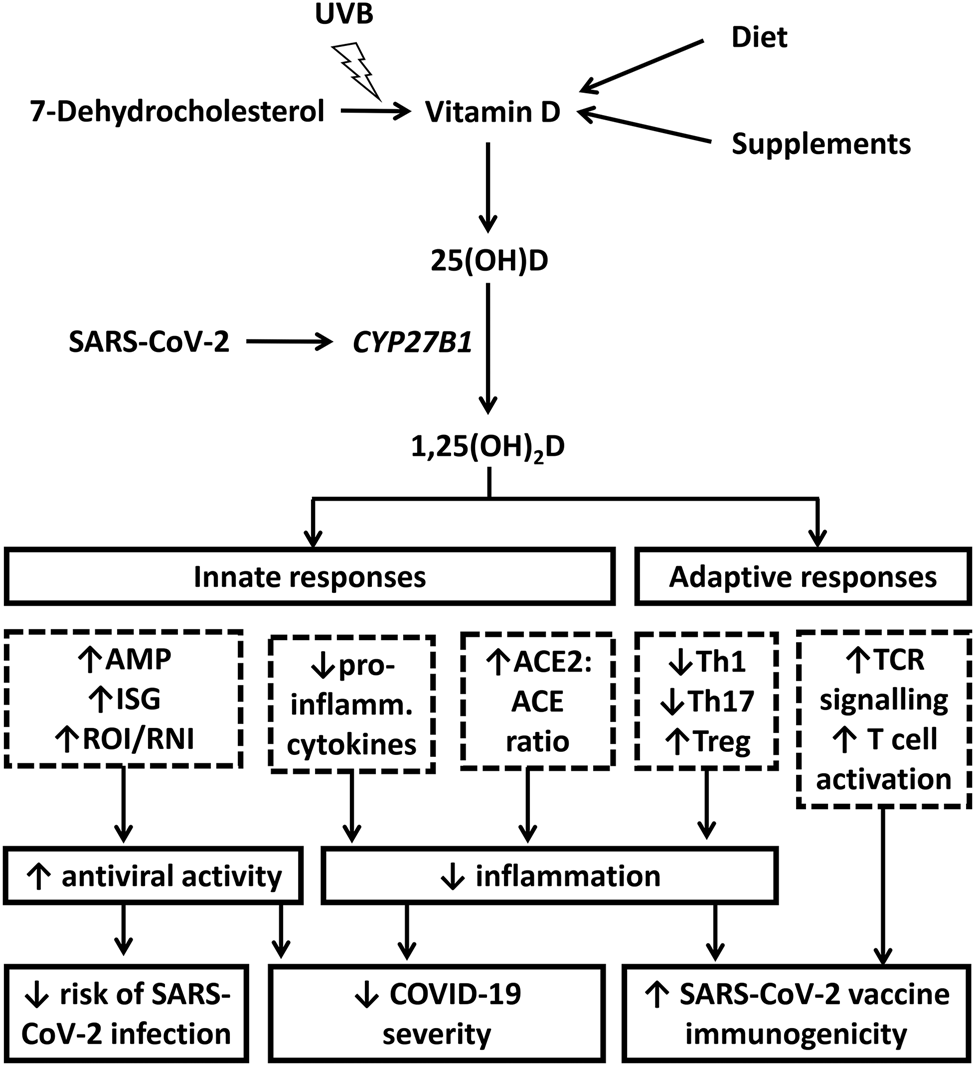
Fig. 2. Putative immunomodulatory actions of vitamin D in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Cutaneous synthesis of vitamin D from 7-dehydrocholesterol is stimulated following exposure to ultraviolet B (UVB) radiation in sunshine; alternative sources are from oral intake of foods or supplements containing vitamin D. ‘Parent’ vitamin D from any of these sources is converted to 25-hydroxyvitamin D [25(OH)D, the major circulating metabolite and measure of vitamin D status], primarily by the liver. SARS-CoV-2 ligates pattern recognition receptors to induce expression of the 25(OH)D hydroxylase CYP27B1 in pulmonary epithelium and leucocytes, which catalyses conversion of 25(OH)D to the active vitamin D metabolite 1,25-dihydroxyvitamin D [1,25(OH)2D]. 1,25(OH)2D3 upregulates antiviral effector mechanisms (expression of antimicrobial peptides [AMP], interferon-stimulated genes [ISG] and generation of reactive oxygen and nitrogen intermediates [ROI, RNI]) with potential to reduce susceptibility to infection and severity of disease. It also exerts anti-inflammatory actions by regulating NF-κB and mitogen-activated protein kinase (MAPK) pathways to reduce expression and secretion of pro-inflammatory cytokines; by increasing the ratio of angiotensin converting enzyme 2 [ACE2] to ACE); and by regulating adaptive responses to inhibit differentiation of null T helper (Th) cells towards type 1 or type 17 phenotypes and to promote their differentiation towards a T regulatory (Treg) phenotype. In the context of active coronavirus disease 2019 (COVID-19), these anti-inflammatory actions have potential to reduce disease severity associated with cytokine storms. In the context of vaccination, they may augment development of antigen-specific immunity. Finally, 1,25(OH)2D may also support classical T cell receptor (TCR) signalling and T cell activation by inducing phospholipase C-gamma 1 (PLC-γ1) in naïve T cells. These actions would also be expected to support development of antigen-specific immunity following vaccination.
Findings from a number of recent mechanistic studies support the relevance of these actions for host responses to SARS-CoV-2(Reference Martineau and Cantorna15). First, in keeping with predictions from an in silico study(Reference Lokhande, Banerjee and Swamy16), the vitamin D-inducible antimicrobial peptide cathelicidin LL-37 has been shown to inhibit SARS-CoV-2 attachment by blocking both the receptor-binding domain of S1 and the ligand-binding domain of ACE2(Reference Wang, Wang and Li17). Secondly, a study in transgenic mice expressing human ACE2 has shown that SARS-CoV-2 induces expression of both CYP27B1 and CYP24A1, with potential implications both for conversion of 25(OH)D to 1,25(OH)2D and for catabolism of 25(OH)D and 1,25(OH)2D via 24-hydroxylation(Reference Arora, Nicols and Patel18). In this study, prophylactic administration of high-dose vitamin D3 increased expression of type I interferons and reduced inflammation in the lung following SARS-CoV-2 infection, although this was not associated with a survival benefit(Reference Arora, Nicols and Patel18). Potential relevance of these findings for human disease is supported by the observation that SARS-CoV-2-infected human bronchial epithelial cells express pro-inflammatory genes predicted to be vitamin D-modifiable(Reference Ahmed19). Studies of leucocytes isolated from patients with COVID-19 also report higher expression of vitamin D-repressible genes encoding T helper 1 cytokines than those of controls, associated with reduced vitamin D receptor expression(Reference Taheri, Rad and Hussen20–Reference George, Amjesh and Paul22). However, a case study of COVID-19 arising in a family lacking functioning vitamin D receptor reported a mild disease course and normal development of antigen-specific cellular and humoral immune responses to SARS-CoV-2(Reference Kongsbak-Wismann, Al-Jaberi and Schmidt23). Taken together, these observations suggest that vitamin D signalling may have a role in regulating SARS-CoV-2-induced inflammation, but that it may not be a pre-requisite for averting severe outcomes or mounting effective adaptive immune responses.
Observational epidemiological studies
Observational studies in this field can be classified into three groups, according to whether they investigate outcomes relating to (1) COVID-19 severity, (2) susceptibility to SARS-CoV-2 infection or (3) immunogenicity of vaccination against SARS-CoV-2. Each will be considered in turn.
Observational studies investigating coronavirus disease 2019 severity
Numerous hospital-based cross-sectional, case-control and longitudinal studies have investigated potential associations between low circulating 25(OH)D concentrations and severity of COVID-19. Although some have yielded null results, the majority show positive associations, and meta-analyses of their findings consistently report statistically significant associations between vitamin D deficiency at hospital admission and increased risk of adverse outcomes including mortality and requirement for intensive care unit admission and ventilatory support(Reference Pereira, Dantas Damascena and Galvao Azevedo24–Reference D'Ecclesiis, Gavioli and Martinoli29). While such associations may be causal, two alternative explanations should be considered, as illustrated in Fig. 3. In one scenario, reverse causality may operate, such that reduced 25(OH)D concentrations arise as a consequence of severe COVID-19. This may arise via dysregulated vitamin D metabolism, since SARS-CoV-2 is recognised to induce expression of CYP24A1, the major catabolic enzyme that may 24-hydroxylate 25(OH)D or 1,25(OH)2D to biologically inactive metabolites(Reference Arora, Nicols and Patel18). Alternatively, such associations may arise as a result of residual or unmeasured confounding by factors associating both with increased risk of vitamin D deficiency and with increased risk of severe COVID-19; these may include older age, Black or South Asian ethnicity, obesity or winter season. Mendelian randomisation studies are less open to confounding or reverse causality than other observational study designs(Reference Davey Smith and Hemani30). Accordingly, the fact that these have consistently shown no association between adverse clinical outcomes of COVID-19 and lower genetically predicted 25(OH)D levels(Reference Butler-Laporte, Nakanishi and Mooser31–Reference Cui and Tian34) raises the possibility that the associations reported in cross-sectional, case-control and longitudinal studies may not be causal.

Fig. 3. Potential explanations for observed associations between vitamin D deficiency and severe coronavirus disease 2019 (COVID-19). (1) Causation: vitamin D deficiency may increase susceptibility to severe COVID-19 via attenuation of antiviral and anti-inflammatory responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). (2) Reverse causation: severe COVID-19 may reduce circulating 25-hydroxyvitamin D [25(OH)D] concentrations, via upregulation of vitamin D catabolism and/or reduction in concentrations of plasma proteins that bind 25(OH)D in the circulation. (3) Confounding: factors including older age, obesity, Black or South Asian ethnicity and winter season may independently associate with increased susceptibility to both vitamin D deficiency and severe COVID-19.
Observational studies investigating susceptibility to severe acute respiratory syndrome coronavirus 2 infection or coronavirus disease 2019
Several population-based cross-sectional and longitudinal studies have investigated potential associations between circulating 25(OH)D concentrations or vitamin D supplement use and risk of incident SARS-CoV-2 infection or COVID-19. Their findings are heterogeneous, with some reporting associations between lower vitamin D status and increased susceptibility to infection(Reference Merzon, Tworowski and Gorohovski35), and others reporting null results(Reference Holt, Talaei and Greenig36). As for studies investigating disease severity, meta-analyses of susceptibility studies have yielded consistent protective associations between higher baseline vitamin D status and reduced risk of incident disease(Reference Kaya, Pamukcu and Yakar26,Reference Dissanayake, de Silva and Sumanatilleke27) , but Mendelian randomisation studies have yielded null results(Reference Butler-Laporte, Nakanishi and Mooser31–Reference Cui and Tian34).
Observational studies investigating severe acute respiratory syndrome coronavirus 2 vaccine immunogenicity
Four observational studies have investigated associations between vitamin D status and SARS-CoV-2 vaccine immunogenicity. These have yielded conflicting results: two report higher post-vaccination titres of anti-spike antibodies in individuals using vitamin D supplements or having higher circulating 25(OH)D concentrations(Reference Jolliffe, Faustini and Holt37,Reference Piec, Cook and Dervisevic38) , but two others have yielded null findings(Reference Chillon, Demircan and Heller39,Reference Parthymou, Habeos and Habeos40) .
Randomised controlled trials
RCTs in this field can be classified into three groups, according to whether they investigate (1) effects of therapeutic vitamin D in patients with established COVID-19; (2) effects of prophylactic vitamin D to reduce risk or severity of incident COVID-19 in healthy subjects or (3) effects of adjunctive vitamin D administered prior to SARS-CoV-2 vaccination to boost vaccine immunogenicity and efficacy (Fig. 1). Each is considered in turn next.
Randomised controlled trials of therapeutic vitamin D to reduce severity of coronavirus disease 2019
Table 1 summarises findings of twelve RCTs investigating therapeutic effects of vitamin D in patients with COVID-19 that have reported to date. They are diverse with respect to study design (five are placebo-controlled, seven are open-label or single-blind), sample size (ranging from 30 to 543 participants), the nature of the intervention [nine investigate vitamin D3, two investigate 25(OH)D3 and one investigates 1,25(OH)2D3] and primary outcomes (mortality, duration of hospital stay, intensive care requirement, resolution of symptoms and viral clearance). Their findings are also heterogeneous: eight trials report null results, while four report favourable effects of the intervention on a primary or co-primary outcome. Perhaps the most striking positive result comes from an open-label trial of oral 25(OH)D administration conducted in seventy-six adults hospitalised for treatment of COVID-19 in Spain, which reported that just 2 % of participants randomised to intervention were admitted to intensive care, as compared with 50 % of those randomised to control(Reference Entrenas Castillo, Entrenas Costa and Vaquero Barrios41). However, this study was at high risk of bias, due to imbalance in baseline characteristics and its open label design, since knowledge of allocation could have influenced physicians' decision to admit participants to intensive care. Moreover, background therapy (azithromycin and hydroxychloroquine) was unconventional, compromising generalisability of results to patients receiving current standards of care. The majority of larger well-conducted RCTs have not demonstrated sustained or consistent benefits of vitamin D on mortality, intensive care requirement or duration of hospital stay(Reference Murai, Fernandes and Sales42–Reference Mariani, Antonietti and Tajer45).
Table 1. Randomised controlled trials of vitamin D3 or its hydroxylated metabolites in the treatment of coronavirus disease 2019 (COVID-19)

1,25(OH)2D3, calcitriol; 25(OH)D3, calcidiol; aHR, adjusted hazard ratio; ICU, intensive care unit; RT-PCR, reverse transcription PCR.
Randomised controlled trials of prophylactic vitamin D to reduce risk of incident coronavirus disease 2019
Two RCTs investigating effects of prophylactic vitamin D have also reported, with contrasting results. A phase 2 placebo-controlled RCT in 321 healthcare workers in Mexico, conducted before roll-out of SARS-CoV-2 vaccination, reported a strong protective effect of daily oral administration of 100 μg vitamin D3 for 1 month against incident SARS-CoV-2 infection(Reference Villasis-Keever, Lopez-Alarcon and Miranda-Novales46). This finding surprised many, given that the duration of the intervention (1 month) was insufficient for participants in the intervention arm to experience a large increase in circulating 25(OH)D concentrations. By contrast, an open-label pragmatic phase 3 RCT in 6200 UK adults conducted during SARS-CoV-2 vaccine roll-out showed no effect of implementing a test-and-treat approach to correction of sub-optimal vitamin D status via daily oral administration of either 20 or 80 μg vitamin D3 over 6 months(Reference Jolliffe, Holt and Greenig47). Interpretation of this result is complicated by the pragmatic nature of this trial, which allowed for consumption of vitamin D supplements among participants randomised to its control arm; however, a sensitivity analysis excluding data from control arm participants who took off-trial supplements also yielded a null finding. Results from placebo-controlled phase 3 trials of prophylactic vitamin D and cod liver oil (clinicaltrials.gov refs NCT04609423, NCT04483635 and NCT04536298) are pending, and these should clarify whether vitamin D supplements can influence risk or severity of COVID-19.
Randomised controlled trials of pre- or peri-vaccination vitamin D to augment immunogenicity of severe acute respiratory syndrome coronavirus 2 vaccines
Three sub-studies nested within the CORONAVIT trial have investigated potential effects of vitamin D supplementation on SARS-CoV-2 vaccine efficacy and immunogenicity(Reference Jolliffe, Vivaldi and Chambers48). The first (n 2823) investigated effects of vitamin D supplementation on risk of breakthrough SARS-CoV-2 infection following two doses of SARS-CoV-2 vaccine. The second (n 1864) investigated effects of vitamin D supplementation on titres of combined immunoglobulin G, immunoglobulin A and immunoglobulin M anti-spike antibodies in eluates of dried blood spots collected after SARS-CoV-2 vaccination. The third (n 101) investigated effects of vitamin D supplementation on neutralising antibody and cellular responses in venous blood samples collected after SARS-CoV-2 vaccination. All yielded null results.
Conclusions
A substantial body of evidence relating to potential effects of vitamin D on risk and severity of COVID-19 has accumulated since the start of the COVID-19 pandemic. Laboratory investigations have demonstrated potential mechanisms by which 1,25(OH)2D may favourably modulate host responses to SARS-CoV-2, and meta-analyses of data from cross-sectional, case-control and longitudinal studies have reported consistent associations between lower vitamin D status and increased risk or severity of COVID-19. By contrast, Mendelian randomisation studies testing for associations between genetically predicted circulating 25(OH)D concentrations and COVID-19 outcomes have yielded null results, raising the possibility that positive findings from observational epidemiological studies may have arisen because of reverse causality or confounding. RCTs of vitamin D for the treatment or prevention of COVID-19 reporting to date have not yielded consistent evidence of benefit. Results of further trials are awaited, but current evidence is insufficient to support the use of vitamin D supplements for prevention or treatment of COVID-19.
Financial Support
None.
Conflicts of Interest
A. R. M. declares receipt of funding in the past 36 months to support vitamin D research from companies that manufacture or sell vitamin D supplements (Pharma Nord Ltd, DSM Nutritional Products Ltd, Thornton & Ross Ltd and Hyphens Pharma Ltd); receipt of vitamin D capsules for clinical trial use from Pharma Nord Ltd, Synergy Biologics Ltd and Cytoplan Ltd; support for attending meetings from companies that manufacture or sell vitamin D supplements (Pharma Nord Ltd and Abiogen Pharma Ltd); receipt of a consultancy fee from DSM Nutritional Products Ltd; receipt of a speaker fee from the Linus Pauling Institute; participation on Data and Safety Monitoring Boards for the VITALITY trial (Vitamin D for Adolescents with HIV to reduce musculoskeletal morbidity and immunopathology, Pan African Clinical Trials Registry ref PACTR20200989766029) and the Trial of Vitamin D and Zinc Supplementation for Improving Treatment Outcomes Among COVID-19 Patients in India (Clinical-Trials.gov ref NCT04641195); and unpaid work as a Programme Committee member for the Vitamin D Workshop.
Authorship
The author had sole responsibility for all aspects of preparation of the present paper.







