As we experience population ageing more of us can expect to reach and enjoy our old age. This demographic shift should be seen as a time of opportunity; however, as a result, we are also experiencing an increase in age-related diseases. The global incidence of dementia is increasing at a rate of one new case every 3 s, with associated medical, social and healthcare costs far exceeding the capacity of most countries(1, Reference Comas-Herrera, Wittenberg and Pickard2). Dementia is a syndrome, usually progressive and chronic in nature, in which there is deterioration in cognitive function beyond what might be expected from normal ageing(3). Due to the degenerative nature of the disease, sufferers lose their ability to perform routine tasks, experience poor quality of life and a loss of autonomy. In 2017, Public Health England reported dementia to be the leading cause of death in older adults in England, overtaking CVD, stroke and lung cancer for the first time. Similar trends have been expressed globally(4), which are largely driven by our increasing older adult population, longer life expectancies and improved diagnostic procedures(Reference Ferri, Prince and Brayne5, Reference Lutz, Sanderson and Scherbov6). In the absence of curative treatments, the focus on maintaining cognitive health, reducing the risk of developing dementia and delaying the onset is now a key priority for public health authorities and governments(Reference Dehnel7).
Dementia risk increases with advancing age, family history and genetic factors, for example, carriers of ApoE ε4 genotype. Dementia is of course, not an inevitable consequence of older age. Several modifiable lifestyle factors have been identified including CVD, diabetes, smoking and obesity(Reference Barnes and Yaffe8). Indeed modifiable factors may promote resilience in ApoE ε4 gene carriers(Reference Kaup, Nettiksimmons and Harris9, Reference Schöttker, Jorde and Peasey10). A role for vitamin D in the aetiology of cognitive impairment and dementia is plausible, supported by substantial mechanistic and epidemiological data, although intervention studies remain sparse. Vitamin D is a steroid hormone, which directly or indirectly regulates thousands of genes acting mainly via the vitamin D receptor (VDR)(Reference Holick11, Reference Nagpal, Na and Rathnachalam12). The VDR is expressed on multiple tissue sites, including the brain, cardiovascular and musculoskeletal systems where it acts on the expression of several gene target products as well as a range of non-genomic effects. An estimated one billion people globally are vitamin D insufficient which provides an opportunity for intervention(Reference Holick11, Reference Lips13). The aim of the present review is to discuss vitamin D in ageing and examine the evidence for the potential role of vitamin D deficiency and brain health and possible levels likely to support cognitive performance in community-dwelling older adults. Furthermore, we aim to consider the complexities of investigating the effects of vitamin D on cognitive outcomes in ageing.
Vitamin D physiology: a focus on ageing
Vitamin D metabolism
Vitamin D is a precursor of the active form 1,25-dihydroxyvitamin D (calcitriol) and is present in two forms; vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol). The main source in human subjects is via action of solar ultraviolet-B (UV-B) radiation (270–300 nm) on skin, converting 7-dehydrocholesterol (provitamin D3) into pre-vitamin D3, which is then rapidly converted to vitamin D3. Vitamin D2 is produced via UV-B radiation on plant sources such as mushrooms and yeast. Natural dietary sources of vitamin D3 are few and include fish liver oils, oily fish, egg yolks and some fortified foods (e.g. some dairy and breakfast cereals) and supplements. Irrespective of how it is acquired, vitamin D offers limited function until it has been activated, a process which requires two hydroxylation steps(Reference Bouillon, Carmeliet and Daci14, Reference DeLuca15). Firstly in the liver by a number of enzymes but primarily vitamin D325-hydroxylase (CYP2RA)(Reference Schuster16), which converts it to the inactive precursor, 25-hydroxyvitamin D (25(OH)D), the prominent circulating form used to determine vitamin D status in human subjects. The second hydroxylation step occurs in the tubular cells of the kidney, by action of the enzyme 25vitamin D3-1α-hydroxylase (CYP27B1), to the biologically active metabolite, 1,25-dihydroxyvitamin D3. This active metabolite is an important modulator of calcium and phosphate homeostasis, importantly; however, both the activating enzyme CYP27B1 and the active form of vitamin D, 1,25-dihydroxyvitamin D3, are present in many non-renal tissues including the human brain, immune cells, cardiovascular systems and pancreatic islets. Due to the ability to be synthesised endogenously, vitamin D is widely accepted not as a classical vitamin but as a steroid hormone, furthermore, its synthesis in the human brain has led to it being commonly referred to as a neurosteroid(Reference Holick17).
Factors influencing vitamin D status in ageing
Several factors influence vitamin D status including skin pigmentation, use of sunscreen or concealing clothing, season, latitude, being older or institutionalised, obesity, malabsorption, renal and liver disease and medication use. In general, UV-B radiation on skin is the main source of vitamin D for human subjects. In countries north of the equator (40–60°N), vitamin D UV-B doses are inadequate for 6 months of the year (October–March)(Reference Webb, Kline and Holick18). Many other environmental factors implicate vitamin D UV-B dose availability including the ozone layer, cloud cover, air pollution, surface reflection and altitude(Reference Engelsen19). With advancing age skin integrity decreases; skin thinness and a reduction in transdermal cholesterol reduce the efficiency of UV-B vitamin D production, as much as 50 % in older adults compared with younger adults(Reference MacLaughlin and Holick20). Behavioural and social changes seen with advancing age further limit cutaneous vitamin D production. Less time spent outdoors due to ill health or limited mobility (institutionalised or homebound), medication use (loop diuretics, statins, glucocorticoids), changes in body composition (increase in fat and decrease in muscle), sun avoidance (melanoma risk), reduced skin exposure (clothing and colder temperatures) and sunscreen use are all significant barriers that contribute to inadequate UV-B vitamin D production in older adults(Reference de Jongh, van Schoor and Lips21–Reference Wortsman, Matsuoka and Chen25).
The UK Scientific Advisory Committee on Nutrition recommends an intake of 10 µg/d (400 IU/d) vitamin D for older adults, a target hard to achieve by dietary contribution alone unless oily fish is eaten daily. Experts in the UK and Ireland recommend that during the winter months and for those at risk of deficiency, vitamin D supplementation should be considered(26, 27). Evidence suggests vitamin D supplement uptake is typically low in the population, including older adults(Reference Cashman, Muldowney and McNulty28). For the most part, dietary sources are limited and not generally consumed in adequate amounts by older adults(Reference Cashman, Muldowney and McNulty28, Reference Cashman, Hill and Lucey29), with an exception for those residing in countries where vitamin D food fortification is in place (United States, Canada, Sweden and Finland). The effects of age on intestinal absorption and decreased renal function may hinder vitamin D metabolism and availability(Reference Kinyamu, Gallagher and Balhorn30, Reference Kinyamu, Gallagher and Petranick31). It has been hypothesised that the metabolism of vitamin D may be reduced due to a decline of intestinal VDR distribution in older adults; however, few studies have been conducted in human subjects, with small samples and conflicting findings(Reference Ebeling, Sandgren and DiMagno32, Reference Kinyamu, Gallagher and Prahl33).
Vitamin D deficiency and ageing
Serum 25(OH)D is accepted as the most accurate measure of vitamin D status; however, there is less agreement on how to define deficiency in the general population or specifically to older adults. The Institute of Medicine advocate serum 25(OH)D concentrations ≥50 nmol/l (≥20 ng/ml) for bone health, and with levels <30 nmol/l (<12·5 ng/ml) considered to be deficient(Reference Ashwell, Stone and Stolte34). The term vitamin D insufficiency is used to describe serum levels of ≥30–50 nmol/l (≥12·5–20 ng/ml)(35). In contrast, the endocrine society recommends serum 25(OH)D levels >75 nmol/l to maximise an effect on bone and muscle metabolism. Suboptimal levels of vitamin D are much more common than clinical toxicity which is rare(Reference Vieth36). Reported vitamin D deficiency rates in published studies vary widely, with considerable methodological differences including the definition of serum 25(OH)D status, sample size, population, sex-specific cohorts, latitude, season and mode of vitamin D assay. Prevalence estimates for vitamin D deficiency in community-dwelling older adults across the European Union and the UK are summarised in (Table 1), along with the specific 25(OH)D cut-off criteria applied to define deficiency. As illustrated in (Table 1), deficiency ranged from 0 to 100 %. Overall, however, it is apparent that suboptimal vitamin D status is widespread in older adults, irrespective of the definition applied. For example, the Health Survey in England reported that 13 % of females and 8 % of males were vitamin D deficient, using the more conservative cut-off of <20 nmol/l(Reference Hirani and Primatesta37), at 5 years follow-up deficiency rates increased to 15 and 9·6 %, respectively(Reference Hirani, Tull and Ali38). When defining criteria is set at <50 nmol/l, 62·5 % of females and 50 % of males were classed as vitamin D deficient in this community-dwelling cohort. However, deficiency rates were almost double in cohorts defined as cognitively impaired(Reference McCarroll, Beirne and Casey39). In a recent study of Irish adults, a 10 % higher prevalence of vitamin D deficiency was reported in adults aged 65 years and older compared to middle-aged adults, 35·7 and 44·0 %, respectively(Reference Cashman, Muldowney and McNulty28).
Table 1. Prevalence of 25-hydroxyvitamin D (25(OH)D) deficiency in community-dwelling adults, aged over 50 years in Europe
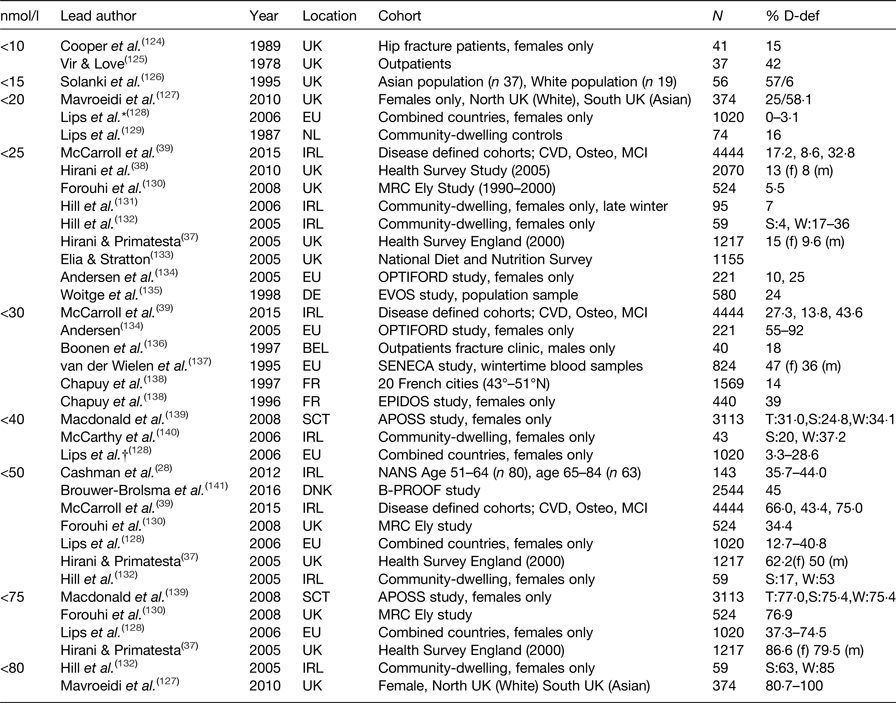
D-def, serum 25(OH)D deficient; T, total; S, summer; W, winter; m, male; f, female.
* Deficiency defined <22·5 nmol/l.
† Deficiency defined <37 nmol/l.
Vitamin D and cognitive function in ageing: considerations for assessment and outcome measures
The concept that cognitive function can be influenced by diet or specific nutrients has garnered interest in recent years. One of the most formidable methodological barriers evident across nutrition and cognition studies is the heterogeneity in cognitive assessments used, which will ultimately influence the outcome and the interpretation of the findings.
The term cognitive function refers to a variety of brain functions and processes which include receiving external information, processing this internally and responding with a behaviour. It can be seen as a hierarchy, going from overall (global) to domain-specific cognitive function. The most commonly used cognitive outcome measure in vitamin D studies are global tests, namely the Mini-Mental State Examination, and consequently, most associations were seen with a global measure(Reference Brouwer-Brolsma, Feskens and Steegenga40–Reference Wilson, Houston and Kilpatrick44). Whilst this clinical screening test is widely used, it has limited use in research for detecting subtle changes in cognitive function in response to short-term interventions, such as vitamin D supplementation trials(Reference Spencer, Wendell and Giggey45). Domain-specific functions include memory, executive functioning, attention, perceptual functions, psychomotor abilities and language skills. Domain-specific measures are useful but reliance on a single domain assessment may lead to cognitive changes going undetected due to intra-individual variability(Reference Petersen46). Assessing cognitive function is complex and the importance of utilising multiple domains and assessing further sub-processes within each domain has been highlighted in recent years(Reference Fisk, Merry and Rockwood47).
Inter-individual performance in different domains vary and each individual has a unique profile of strengths and weaknesses in different domains(Reference Kanai and Rees48), so it is not explicit to define people on global function or domain-specific function alone. Performing well on one domain is often dependent on traits of another cognitive process or domains, for example, retaining new information in a memory test is highly dependent on attention(Reference Carriere, Cheyne and Smilek49). Studies which report having assessed participants’ cognitive function but use only a single domain outcome measure should be interpreted with caution unless the hypothesis predicts a specific effect on memory; even then including a mix of tests will better determine whether the predicted memory-specific function was indeed affected specifically.
Other factors related to cognitive assessments
Each domain is highly influenced by other mental factors, a psychological concept called central arousal which describes internal states such as mental fatigue and needs to be considered when assessing cognitive performance(Reference Eysenck, Derakshan and Santos50). Mood, sleep, anxiety and depression are highly correlated with cognitive performance and can significantly alter the outcome(Reference Conroy, Golden and Jeffares51, Reference Simpson, Maylor and McConville52).
The assessment of cognitive function is further complicated in defining the cognitive status of study participants, a distinct difference must be acknowledged when considering cognitive changes which are age-related and those which are a result of disease. Furthermore, inter-individual variability in the form and severity of cognitive decline is largely influenced by education and health-related factors. To accurately assess changes in cognitive status, these factors must also be considered, whilst acknowledging that older adults vary widely in the extent to which they are affected by these changes(Reference Kanai and Rees48). For example, to our knowledge, no studies of vitamin D status and cognitive performance considered assessment of pre-morbid IQ, which is highly correlated with most cognitive tests, since decline is essentially relative to pre-morbid ability(Reference Barker-Collo, Bartle and Clarke53), a score within the ‘normal’ range can, in fact, represent cognitive decline for an individual with high levels of pre-morbid functioning.
Types of outcome measures commonly used in vitamin D and cognition research
Neuropsychological performance tests are the methods most commonly used in vitamin D and cognition studies. Cognitive function can be assessed by a number of validated pen-and-paper or computer-based tests. Direct measures of brain location and effectiveness of many cognitive processes can be assessed using electroencephalograms, or assessment of brain activation associated with specific domains of cognitive function through functional MRI, often considered the gold standard, however, like all tests is subject to limitations. The relationship between neuronal activity and functional ability is not fully understood(Reference Poldrack and Wagner54) and their use in measuring nutritional effects should be interpreted carefully. Emerging mobile technologies which obtain real-time information regarding abilities to perform activities of daily living, which are highly correlated with neuropsychological tests, may change the way cognitive research is conducted, in way of preventative strategies for dementia(Reference Allard, Husky and Catheline55).
As the evidence for an underlying link between vitamin D and cognitive performance remains inconclusive, the best approach is to include a battery of cognitive tests that cover a variety of domains; this may help identify the specific cognitive processes involved. Several large-scale ageing studies incorporate a comprehensive battery of validated cognitive tests(Reference Hannigan, Coen and Lawlor56, Reference Kenny, Coen and Frewen57); their use in cognitive studies on vitamin D may be helpful. However, the logistics, time and practicalities of applying comprehensive cognitive batteries in vitamin D studies may be a barrier. Recently we reported preliminary findings that this was feasible and acceptable in adults aged 60 years and older, at three time points over a 6-month period(Reference Aspell, Lawlor and O'Suillivan58).
Epidemiological evidence; low vitamin D status and cognitive performance in healthy older adults
In the decade succeeding the discovery of the VDR and 1-α-hydroxylase in the human brain, a plethora of evidence for a relationship between serum 25(OH)D and cognitive function has been presented. Data from healthy older adults without known cognitive impairment provides comparable evidence in terms of evaluating the contribution of vitamin D and successful ageing; however, this information is less plentiful. To date, the majority of cross-sectional studies support an association between hypovitaminosis D and poorer cognitive performance. Yet many use only brief measures of cognitive function or adjust for few or limiting confounding factors. A recent cross-sectional study demonstrated that older adults with low 25(OH)D status (<30 nmol/l) performed significantly worse than those with levels >75 nmol/l, including greater processing speed and mental flexibility(Reference van Schoor, Comijs and Llewellyn43). This evidence was drawn from a well-designed large national study of ageing conducted in Amsterdam, which employed an in-depth cognitive assessment and gathered detailed demographic and lifestyle information. In contrast, the largest cross-sectional study conducted to date found no association between 25(OH)D status and cognitive function(Reference Tolppanen, Williams and Lawlor59), comprising 4831 participants of the National Health and Nutrition Examination Survey. The result may be attributed to the single memory outcome measure used to assess cognitive function. Three large population cohorts have since demonstrated a significant relationship between low 25(OH)D status (typically <30 nmol/l) and poorer cognitive performance, using comprehensive global and domain-specific outcomes, compared with adequate 25(OH)D levels (typically >75 nmol/l)(Reference van Schoor, Comijs and Llewellyn43, Reference Wilson, Houston and Kilpatrick44, Reference Bartali, Devore and Grodstein60). Other studies have demonstrated a relationship also, however, methodological issues were noted, with small sample sizes, limited analysis of confounding factors and single measures of cognitive performance(Reference Brouwer-Brolsma, Feskens and Steegenga40, Reference Brouwer-Brolsma, van de Rest and Tieland61–Reference Seamans, Hill and Scully63). Cross-sectional studies are considered to provide weak evidence, due to the known issue of reverse causality, in that poorer cognitive performance and the onset of dementia may influence vitamin D concentrations through behavioural and dietary changes.
Longitudinal studies suggest that vitamin D deficiency is associated with an increased risk of cognitive impairment and incidence of dementia and Alzheimer's disease (AD)(Reference Littlejohns, Henley and Lang64). One such study conducted with 10 186 older adults followed up after 30 years, revealed that those with 25(OH)D levels <25 nmol/l had a greater combined risk of developing AD than those with levels >50 nmol/l(Reference Afzal, Bojesen and Nordestgaard65). Another prospective study revealed that those with 25(OH)D concentrations at baseline, and at 3- and 6-year follow-up performed worse in global function and executive functioning but not in tasks of attention(Reference Llewellyn, Lang and Langa66). Whilst most studies demonstrate a link between serum 25(OH)D deficiency and poorer cognitive performance or incidence of dementia using a measure of global or domain-specific cognitive function(Reference Balion, Griffith and Strifler67), it is not clear if vitamin D deficiency is a risk factor for cognitive impairments or a result of poorer overall status and ill health(Reference van der Schaft, Koek and Dijkstra68).
Intervention evidence; the effects of vitamin D supplementation on cognitive performance
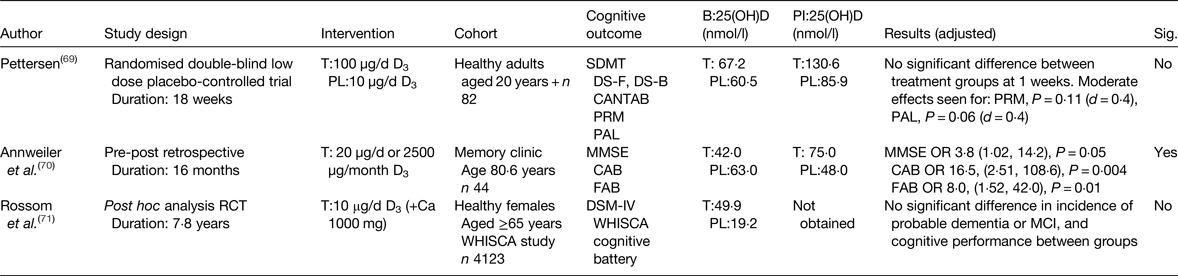
There are limited data from intervention studies. To date, three studies have been published investigating the effect of vitamin D supplementation on cognitive performance in healthy older adults. Overall one of three reported a significant positive effect on cognitive measures (Table 2). Recently, the first prospective intervention, using 50 µg/d (2000 IU) vitamin D3 reported no significant improvement in tasks of visual memory among eighty-two community-dwelling adults(Reference Pettersen69). Of note, was the heterogeneity between participants, as only 30 % of this small sample were aged over 60 years.
Table 2. Intervention studies; effect of vitamin D supplementation and cognitive performance outcomes in healthy older adults

B, baseline; PI, post-intervention; T, treatment; PL, placebo; SDMT, symbol digit modalities test; DS-F, digit span forward; DS-B, digit span backward; CANTAB, Cambridge neuropsychological test automated battery; 25(OH)D, 25-hydroxyvitamin D; PRM, pattern recognition memory; PAL, paired associates learning; MMSE, mini-mental state examination; CAB, cognitive assessment battery; FAB, frontal assessment battery; WHISCA, women's health initiative study of cognitive aging; DSM-IV, diagnostic and statistical manual of mental disorders; MCI, mild cognitive impairment; Sig., significant.
Annweiler et al. (Reference Annweiler, Herrmann and Fantino70), however, reported a positive finding in a small retrospective study (n 44), cognition was measured using a global measure and a behavioural assessment among patients attending an outpatient clinic, without cognitive impairment at baseline(Reference Annweiler, Herrmann and Fantino70). Incomplete 25(OH)D data were available for all participants, so determining optimal levels for cognitive performance are unobtainable from this investigation.
In the largest intervention study conducted to date, in community-dwelling females across the USA, no effect for vitamin D supplementation and incidence of dementia or cognitive performance was seen at follow-up (7·8 years)(Reference Rossom, Espeland and Manson71). Whilst an in-depth cognitive assessment was conducted, the intervention design was poor, the entire study population were open to consuming supplemental vitamin D of 15 µg/d (600 IU/d) during the study period, irrespective of treatment allocation, this left further ambiguity in terms of true treatment effects as serum 25(OH)D concentrations were not measured at the end of the study, providing no evidence for optimal serum 25(OH)D levels and cognitive function.
Preliminary work from our group shows no effect on global function and domain-specific tasks of executive function and attention, following 6-month vitamin D3 intervention of 50 µg/d (2000 IU/d) in healthy older adults; further work on domain-specific cognitive outcomes is ongoing(Reference Aspell, Lawlor and O'Suillivan58). Overall evidence from intervention studies is in its early stages, whilst published studies to data are inconclusive. This may be attributable to study design; nevertheless, these shortcomings have helped inform well-designed interventions(Reference Bischoff-Ferrari72), findings of which are likely to be available in the near future.
Vitamin D and Alzheimer's disease
Other interventions have been conducted in participants with mild–moderate AD and institutionalised older adults; however both showed no effect, were of short duration and add little in terms of preventable, risk factors for AD. One reported an improvement in attention and reaction times in 139 ambulatory subjects who were supplemented with vitamin D for 6 months(Reference Dhesi, Jackson and Bearne73). The other study was retrospective in design, non-blinded and involved only thirty-two participants presenting with subjective memory complaint, had a short duration of treatment of 28 d and used only one limited test of cognition(Reference Przybelski and Binkley74). Several studies report low levels of 25(OH)D in the community in patients with AD(Reference Buell, Dawson-Hughes and Scott75–Reference Sato, Asoh and Oizumi77).
Vitamin D and cognitive decline: mechanisms behind the link
Most research in this field is primarily derived from animal studies and key findings are detailed later. For a full overview of the literature examining the link between vitamin D in ageing and cognitive health, see Eyles et al. and Groves et al. (Reference Eyles, Burne and McGrath78, Reference Groves, McGrath and Burne79). It is hypothesised that vitamin D exerts its effects via genomic and non-genomic pathways(Reference Fernandes de Abreu, Eyles and Feron80–Reference Ramagopalan, Heger and Berlanga82). Exact mechanisms are unclear, but evidence suggests that it may protect against cognitive dysfunction through its effect on neuroprotection, neurotransmission, synaptic plasticity, immune modulation, neuronal calcium regulation and enhanced nerve conduction(Reference Brown, Bianco and McGrath83–Reference Garcion, Wion-Barbot and Montero-Menei85), with secondary protective effects on vascular systems and modulation of vascular risk factors(Reference Wang, Pencina and Booth86).
Vitamin D metabolism and the central nervous system
The discovery of 1α-hydroxylase and metabolic pathways for vitamin D in the human brain(Reference Eyles, Smith and Kinobe87) and cerebrospinal fluid support a localised catabolic pathway for vitamin D in the central nervous system. The three enzymes necessary for complete synthesis and catabolism of active vitamin D has also been expressed(Reference Balabanova, Richter and Antoniadis88, Reference Reinhardt and Horst89). Serum 25(OH)D has also been evidenced to cross the blood–brain barrier a characteristic shared by other neurosteroids(Reference Kalueff, Minasyan and Keisala90).
Vitamin D receptor and the central nervous system
The nuclear functions of 1,25-dihydroxyvitamin D3 are mediated through the VDR, a member of the nuclear receptor family identified in over 2700 genomic sites(Reference Ramagopalan, Heger and Berlanga82, Reference Petkovich, Brand and Krust91). Experimental scientists have demonstrated the extensive mapping of the VDR in the rat brain(Reference Feron, Burne and Brown92–Reference Walbert, Jirikowski and Prufer95), showing a similar distribution in human brain tissues(Reference Eyles, Smith and Kinobe87), with the earliest evidence in post-mortem brains of AD and Huntington's disease patients(Reference Eyles, Smith and Kinobe87, Reference Sutherland, Somerville and Yoong96). The identification of its distribution in neuronal and glial cells in the human brain followed thereafter, supporting a functional role in light of local production of active vitamin D in the human brain(Reference Eyles, Smith and Kinobe87). Areas identified in both animal and human brain tissue show similar patterns(Reference Stumpf, Sar and Clark94), in peripheral neurons,(Reference Tague and Smith97) and in several cell types of the central nervous system(Reference Prufer, Veenstra and Jirikowski93, Reference Walbert, Jirikowski and Prufer95, Reference Cornet, Baudet and Neveu98). The action of 1,25-dihydroxyvitamin D3 upon binding to the VDR has been linked with a diverse range of biological systems such as immune modulation, cell growth and cell differentiation all of which impact brain function via interaction with target genes(Reference Gronemeyer, Gustafsson and Laudet99, Reference Omdahl, Morris and May100).
Vitamin D and neuroprotection
Animal and cell culture evidence suggest that vitamin D may protect the structure and integrity of neurons through detoxification pathways and neurotrophin synthesis(Reference Wang, Wu and Cherng101). Vitamin D is known to affect the expression of three of the four neurotrophins; nerve growth factor, glial-derived nerve factor and neurotrophin 3(Reference Neveu, Naveilhan and Baudet102). Treatment of vitamin D in adult rats resulted in increased glial-derived nerve factor expression and immunoreactivity after dopamine toxicity damage(Reference Sanchez, Lopez-Martin and Segura103). Furthermore, vitamin D3 may attenuate neurotoxicity; administration of calcitriol has been shown to increase levels of antioxidants, such as glutathione in the rat brain which protects oligodendrocytes and the integrity of nerve conduction pathways critical to mental processing(Reference Chen, Lin and Chiu104). The in vitro analysis shows vitamin D treatment inhibits production TNF-α and IL 6, suggesting an anti-inflammatory role(Reference Lefebvre d'Hellencourt, Montero-Menei and Bernard105).
Vitamin D and regulation of intraneuronal calcium
Elevated levels of calcium in the brain leads to neurotoxicity. Three calcium-binding proteins have been shown to be modulated by vitamin D in brain tissues, calbindin, parvalbumin and calretinin(Reference Alexianu, Robbins and Carswell106). All three are widely and uniquely distributed in the adult brain and are believed to serve a neuroprotective role as calcium buffers(Reference Fierro and Llano107) as well as being involved in critical brain functions. In vitamin D-deficient mice, certain calcium channels are shown to be unregulated leading to an increase in Ca2+(Reference Zhu, Zhou and Yang108) and in vitro evidence has shown that vitamin D can down-regulate calcium channels(Reference Brewer, Thibault and Chen109).
Vitamin D and secondary vascular protection
Vascular dementia is the second most commonly occurring type of dementia after AD, though markedly different can also co-exist as ‘mixed dementia’. Vascular dementia is characterised by cognitive dysfunction secondary to ischemic or haemorrhagic brain lesions due to cerebrovascular disease or CVD. This type of brain damage may result from an influx of excitatory amino acids, inflammatory responses and changes in cell function, which result in excessive calcium entry(Reference Román, Tatemichi and Erkinjuntti110). With these changes presents an increase in intracellular nitric oxide production and increased oxidative stress.
Vitamin D may help improve vascular-related brain disease by mediating harmful effects of inflammation, calcium dysregulation and increased oxidative stress. During transient ischemic events, transforming growth factor and glial-derived nerve factor are upregulated in hippocampal cells to promote survival(Reference Garcion, Sindji and Leblondel111). As described earlier, vitamin D enhances innate antioxidative defences by increasing glutathione and glial-derived nerve factor concentrations(Reference Garcion, Sindji and Leblondel111–Reference Wion, MacGrogan and Neveu113). These particular changes were shown to attenuate ischemic brain disease in rodents(Reference Wang, Wu and Cherng101). Furthermore, 1,25 dihydroxyvitamin D inhibits inducible nitric oxide synthetase(Reference Garcion, Nataf and Berod114), an enzyme that is up-regulated during ischaemic events and in patients with AD(Reference Vodovotz, Lucia and Flanders115). It is responsible for generating nitric oxide which is known to cause damage to neurons and oligodendrocytes at high concentrations(Reference Law, Gauthier and Quirion116).
It is plausible that vitamin D may influence vascular-related dementia via indirect mechanisms. Therapeutic intervention with vitamin D regulates blood pressure(Reference Lind, Wengle and Wide117, Reference Pfeifer, Begerow and Minne118), cardiac hypertrophy(Reference O'Connell, Berry and Jarvis119) and plasma rennin activity(Reference Burgess, Hawkins and Watanabe120, Reference Li, Kong and Wei121). There is an inverse relationship between vitamin D levels and congestive heart failure(Reference Zittermann, Schleithoff and Tenderich122) and vitamin D insufficiency is associated with incident CVD(Reference Wang, Pencina and Booth86).
Future directions
All being considered, a functional role for vitamin D in the central nervous system is still not clear but the potential for neuronal and glial cell-specific actions, e.g. proliferation, differentiation and immune modulation are well evidenced in animal models. Research into vitamin D and cognition is likely to benefit from strong collaborations between scientists trained in both disciplines. Whilst much effort is being made to standardise reporting of vitamin D status, similar standarisation of cognitive outcomes in vitamin D studies would progress our understanding. Current guidelines show that daily supplementation of 50 µg/d (2000 IU/d) is acceptable in community-dwelling adults without medical supervision(Reference Hanley, Cranney and Jones123). In countries where mandatory food fortification is in place, fortification levels remain low and therefore contribute no risk for intoxication, even when supplemental D is adhered to. It is warranted to counsel vitamin D supplementation in this population.
Conclusion
Dementia risk is a prominent concern for all individuals, governments and health professionals worldwide. Identification of modifiable risk factors is at the forefront of the ageing research agenda. There is plausible biological evidence for a role of vitamin D status in brain health from animal models. Epidemiological evidence supports this relationship but few randomised controlled trials have been conducted and show inconsistent findings. It is of great importance as vitamin D deficiency is a worldwide pandemic and is seen most commonly in older adults, residing in northerly latitudes and those of non-white ethnicity, particularly during the winter months. We are unable to indicate an optimal 25(OH)D level that may support cognitive function in healthy older adults, it seems at a minimum we should first aim to prevent deficiency through vitamin D public health strategies. There are many unanswered questions regarding a role for vitamin D and cognitive ageing; (1) What, if any, is the optimal level required to support neuroprotection? (2) At what life stage is intervention most likely to optimise brain health? (3) Is the link mediated by other lifestyle factors such as physical function? Well-designed randomised controlled trials using valid comprehensive cognitive assessments are required to determine if raising 25(OH)D concentrations through supplemental vitamin D improves cognitive health and contributes a viable component of lifestyle approaches to maintain brain health.
Financial support
N. A. is supported by a scholarship from the Irish Research Council.
Conflict of interest
None.
Authorship
N. A., B. L. and M. O'S. wrote the manuscript and approved the final draft of the submitted manuscript.