Iodine (I2), present in food as iodide (I−), is mainly found in saltwater fish, iodised salt, molasses, seaweed and the leaves of plants growing close to the sea. Iodine is an essential trace mineral particularly vital for its function in the synthesis of thyroid hormones that aid the regulation of metabolic activities and the development of the central nervous system and the brain. It is also involved, importantly, in a number of physiological processes(1, Reference Zimmermann2). The Dietary Reference Intakes recommend an estimated average requirement of 90 µg/l for children 3–8 years old, 120 µg/l for boys aged 9–13 years and men older than 70 years old, and 150 µg/l for boys aged 14–18 years, girls aged 9–18 years, men aged 19–70 years and women aged 19 years and older. During pregnancy and lactation 220 and 290 µg/l is recommended, respectively(1).
Both iodine deficiency (ID) and excess are associated with adverse health effects(Reference Delange3–Reference Andersson, Takkouche and Egli5). Inadequate intake of iodine causes mental and health disorders, collectively known as iodine deficiency disorders (IDD). IDD include goitre, cretinism, deafness, motor disabilities, hypothyroidism and mental retardation resulting from damage during brain development(Reference Izzeldin, Crawford and Jooste6, Reference Chuot, Galukande and Ibingira7). Beyond the adverse physiological effects, inadequate iodine intake can also result in negative social outcomes. Individuals with goitre may face social discrimination, which can cause psychological tension, reduced opportunity for marriage and lowered participation in social events(Reference Abuye, Berhane and Akalu8, Reference Keno, Ahrens and Lauvai9). Conversely, excessive iodine intake can block thyroid hormone synthesis and release and thus increase the risk of thyroiditis, goitre, hypothyroidism and possibly fatal iodine-induced hyperthyroidism(Reference Izzeldin, Crawford and Jooste6, Reference Medani, Elnour and Saeed10), and may possibly cause autoimmune diseases(Reference Medani, Elnour and Saeed10–13). Balancing iodine intakes is thus necessary since inadequate and excessive intakes lead to adverse health outcomes(Reference Teng, Shan and Teng11, Reference Thomopoulos12). Pregnant women require additional iodine for the development of the fetus and to compensate for the increased iodine excretion during pregnancy(Reference Chinyanga14). Adequate iodine intake is especially important during pregnancy and infancy. ID can cause abortions, stillbirths, perinatal and infant mortality, as well as irreversible brain damage and impaired psychomotor development among children(Reference Chinyanga, Chidede and Machisvo15).
The WHO indicated that 42·6 % of the African population had insufficient iodine intakes in 2004(13). At the same time, thirteen countries had insufficient iodine intake, eleven countries had adequate intake, nine had a risk of high iodine intake and three countries had excessive intakes. Data were not available for twelve of the sub-Saharan Africa (SSA) countries. A decade (1993 to 2003) of trend analyses indicated that the total goitre prevalence increased from 15·6 to 28·3 %(13). More recently, a global report from the Iodine Global Network reported that out of 139 countries, nineteen countries still experienced inadequate iodine intake, 110 countries had optimal intake and ten countries were classified as at risk of excessive intake. This report also indicated that the majority of countries in most of the regions on the African continent, such as Burkina Faso, Burundi, Mali (Western Africa), Mozambique (Southern Africa), South Sudan and Sudan (Northern Africa), showed sufficient intakes of iodine(16). Moreover, a number of African countries are experiencing both inadequate and excess iodine intakes(13, 16, Reference Seal, Creeke and Gnat17). The lack of dietary diversity in the diets of most African countries and the fact that women often become pregnant at an early age, may contribute to inadequate dietary intakes(Reference Keno, Ahrens and Lauvai9, Reference Korkalo, Freese and Alfthan18). ID interventions include food fortification, supplementation and promotion of consumption of iodine-rich foods. Iodine-related interventions mainly target pregnant women, lactating mothers and children because these groups are more vulnerable to IDD, and thus leading to their exposure to/participation in more than one intervention at the same time(Reference Kassim, Ruth and Creeke4, 19).
There have been significant efforts to reduce ID and goitre in the world, especially in Africa. However, several research investigations have found the existence of not only inadequate, but also excess iodine intakes. To date, no Africa-wide review has been carried out about iodine intake or status in SSA. Thus, this paper aims to (1) review the iodine status among populations in SSA until October 2018, and (2) identify populations at risk of excess or inadequate iodine intakes.
Methods
Search strategies
A team of seven members was involved in this review. The peer-reviewed articles were systematically selected but not evaluated for scientific rigour. The principal investigator guided the team members in searching, checking inclusion and exclusion criteria, and data extraction from the selected peer-reviewed articles. Research questions, objectives, search engines and key words aligning with the objectives for searching were decided in several group meetings at the beginning of the review process. All the authors independently reviewed the included articles and analysed them. The final results were the outcome of group discussions of each individual's analysis and consensus in findings.
A systematic and structured search in PubMed, ScienceDirect and Scopus was conducted using an advanced search process in April 2017. The research team made another search in October 2018 to include updated research articles in this review. For this review, six key words were used to identify relevant articles: ‘iodine intake’, ‘iodine status’, ‘excess of iodine’, ‘hyperthyroidism’, ‘hypothyroidism’, ‘Sub-Saharan Africa’ and various combinations of the name of each country situated in SSA. Limitation on the year of published articles was not accounted for as we aimed to assess iodine status over time in SSA. Three authors independently searched articles using the selected archives and transferred all outputs to an Excel spreadsheet. A final Excel spreadsheet of searched outputs was prepared by resolving disagreements and reaching consensus of the outputs.
Screening and selection
The research team initially found a total of 499 outputs during the search for research articles using the selected key words and combinations. Articles included were based on the eligibility criteria, namely: (a) original research article, (b) research conducted on human subjects, (c) research conducted in any part of the SSA countries, (d) published abstract available in English and (e) studies focusing on the assessment of iodine status using urinary iodine concentration (UIC), dietary intake or the prevalence or association of hypothyroidism or hyperthyroidism with iodine intake or status. Studies focusing on unnatural sources of iodine, one subject-based case studies, radiation or medication were excluded in the screening process. All the searched articles were listed in an Excel spreadsheet. Five authors of the team independently screened the titles and abstracts of all the articles to eliminate duplicates and checked for eligibility to be included in this review. Any disagreements among the authors regarding eligibility of articles were resolved through group discussions to reach a consensus.
Extraction of data
Three authors (S. S., Y. Z-N. and U. M.) independently read through all the twenty-three peer-reviewed articles that passed the eligibility criteria, to make sure of the eligibility for inclusion in this review. Afterwards, S. S., Y. Z-N. and U. M. independently read all the articles to extract data. Basic information of the article, study period, study objective/s, target population, study design, sample size, measurement method of exposure and outcome, intervention (if it was an interventional study), confounders, and main results prevalence, or percentage of ID or excess iodine intakes and measures related to association or determinants, were recorded. Key contents of the published articles were extracted and transferred to an Excel spreadsheet. The main results were prepared and discussed for each article. Afterwards, two other authors (B. A. Z A. and W. O-T.) independently reviewed all twenty-three articles and extracted data as part of cross-checking. The research team held group discussions to resolve disagreements to reach a consensus on the extracted data. Fig. 1 represents the flow chart of the selection process for the published articles.
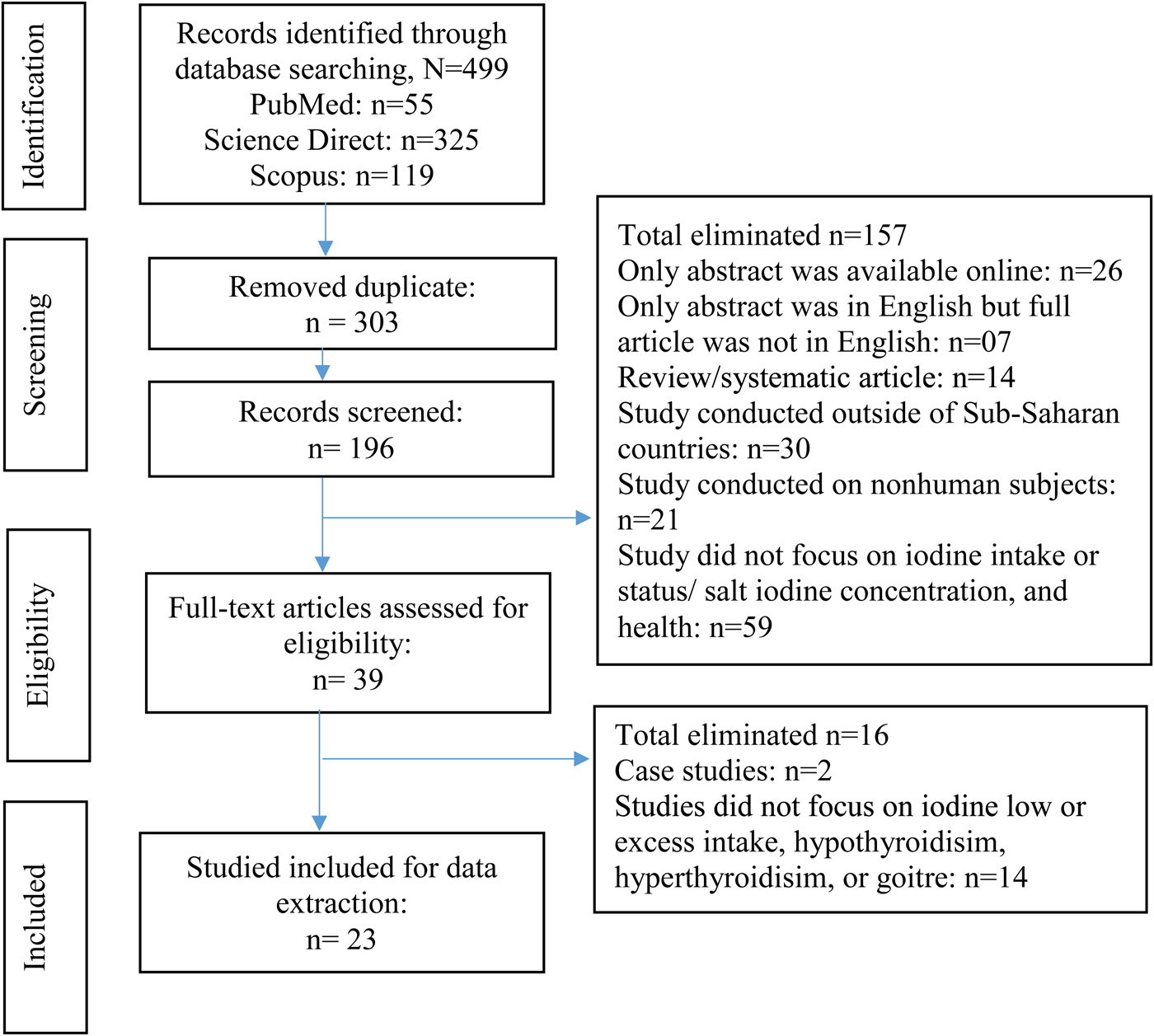
Fig. 1. (Colour online) Flow chart of the article selection process.
Indicators to assess iodine status
The four recommended indicators to assess iodine nutritional status are: UIC or urinary iodine excretion, goitre rate, serum thyroid stimulating hormone level and serum thyroglobulin level(Reference Zimmermann2). UIC is measured using individual urine or urine spot samples(Reference Zimmermann2, Reference Delange, De Benoist and Alnwick20). Goitre rate is measured by visual observation, palpation or through the less-commonly used ultrasonography(Reference Izzeldin, Crawford and Jooste6, Reference Delange, De Benoist and Alnwick20, Reference Benade, Oelofse and van Stuijvenberg21). Serum thyroid stimulating hormone and serum thyroglobulin are thyroid hormones that can be measured in blood samples and used as determinants of thyroid function and iodine nutrition(Reference Zimmermann2, Reference Chinyanga, Chidede and Machisvo15). Other thyroid hormones, such as free thyroxine 4 and free triiodothyronine 3, may also be used as indicators, but are considered less reliable(Reference Zimmermann2). Although total goitre prevalence, UIC, thyroid stimulating hormone, and dietary iodine recall intakes have been used to measure iodine status or iodine intake among general populations(13, 16, Reference Assey, Greiner and Mzee22), analysis of blood and urine samples are the most commonly used methods to estimate iodine intake in a population(Reference Medani, Elnour and Saeed10). Urinary iodine excretion, via the kidneys, is considered an effective biochemical indicator of recent iodine intake(Reference Kassim, Ruth and Creeke4). The cut-off value for IDD is a median UIC level lower than 100 µg/l. IDD is further classified as severe (<20 µg/l), moderate (20–49 µg/l) and mild (50–99 µg/l)(23, 24). If more than 20 % of the UIC levels of a population are below 50 µg/l, it meets one of the requirements for sustainable elimination of IDD(Reference Kassim, Ruth and Creeke4). UIC above 300 µg/l is considered excessive iodine intake. According to the WHO/UNICEF/International Council for Control of Iodine Deficiency Disorders classification, indicators for assessing IDD, the percentage of subjects with UIC less than 50 and 100 µg/l should be less than 50 and 20 %, respectively, in order for the population to be iodine sufficient(23, 24). For the purpose of this review, UIC and goitre results are reported. UIC is less subjective as it measures current iodine states (over the last few days before sampling) whereas a physical goitre reflects a considerably longer history(Reference Chuot, Galukande and Ibingira7).
Results
The twenty-three studies included and information collected in this review were conducted in the following countries: Central Africa (Cameroon, Central African Republic, Democratic Republic of the Congo)(Reference Delange, De Benoist and Alnwick20, Reference Peterson, Legue and Tylleskär25), Eastern Africa (Djibouti, Ethiopia, Kenya, Tanzania)(Reference Kassim, Ruth and Creeke4, Reference Abuye, Berhane and Akalu8, Reference Keno, Ahrens and Lauvai9, Reference Delange, De Benoist and Alnwick20, Reference Assey, Greiner and Mzee22, Reference Farebrother, Zimmermann and Abdallah26, Reference Kassim, Moloney and Busili27), Northern Africa (South Sudan, Sudan)(Reference Izzeldin, Crawford and Jooste6, Reference Chuot, Galukande and Ibingira7, Reference Medani, Elnour and Saeed10, Reference Elnagar, Eltom and Karlsson28, Reference Eltom, Eltom and Elnagar29), Southern Africa (Lesotho, Mozambique, South Africa, Zambia, Zimbabwe)(Reference Chinyanga14, Reference Chinyanga, Chidede and Machisvo15, Reference Korkalo, Freese and Alfthan18, Reference Delange, De Benoist and Alnwick20, Reference Benade, Oelofse and van Stuijvenberg21, Reference Gomo, Allain and Matenga30–Reference Team32) and Western Africa (Benin, Burkina Faso, Cameroon, Ghana, Mali, Nigeria, Togo)(Reference Delange, De Benoist and Alnwick20, Reference Abizari, Dold and Kupka33–Reference Torheim, Granli and Sidibé35). Three studies were conducted in multiple countries(Reference Delange, De Benoist and Alnwick20, Reference Farebrother, Zimmermann and Abdallah26, Reference Delange, Kibambe and Ouedraogo34). None of the articles reported national studies except for one study(Reference Kassim, Moloney and Busili27). The majority of included studies were peer-reviewed and contained sub-national research. Many studies were not recent, ranging from as early as 1986 to only eight studies dated 2010 and later(Reference Kassim, Ruth and Creeke4, Reference Chuot, Galukande and Ibingira7, Reference Keno, Ahrens and Lauvai9, Reference Medani, Elnour and Saeed10, Reference Korkalo, Freese and Alfthan18, Reference Farebrother, Zimmermann and Abdallah26, Reference Kassim, Moloney and Busili27, Reference Abizari, Dold and Kupka33). The most recent studies (n 2) were published in 2017(Reference Keno, Ahrens and Lauvai9, Reference Farebrother, Zimmermann and Abdallah26). Eleven of the studies included had the objective to determine the prevalence of IDD and iodine status of the study populations(Reference Izzeldin, Crawford and Jooste6–Reference Keno, Ahrens and Lauvai9, Reference Chinyanga14, Reference Korkalo, Freese and Alfthan18, Reference Benade, Oelofse and van Stuijvenberg21, Reference Assey, Greiner and Mzee22, Reference Kassim, Moloney and Busili27, Reference Team32, Reference Torheim, Granli and Sidibé35). Five studies measured the impact of uncontrolled and unmonitored salt iodisation on iodine excess(Reference Abuye, Berhane and Akalu8, Reference Delange, De Benoist and Alnwick20, Reference Gomo, Allain and Matenga30, Reference Sebotsa, Dannhauser and Jooste31, Reference Delange, Kibambe and Ouedraogo34). Among these, the purposes of three studies were to determine the prevalence of ID and its relationship with goitre, and other potential goitre-causing risk factors(Reference Abuye, Berhane and Akalu8, Reference Delange, De Benoist and Alnwick20, Reference Gomo, Allain and Matenga30). Six of the studies included iodine status during pregnancy(Reference Kassim, Ruth and Creeke4, Reference Keno, Ahrens and Lauvai9, Reference Chinyanga14, Reference Chinyanga, Chidede and Machisvo15, Reference Farebrother, Zimmermann and Abdallah26, Reference Eltom, Eltom and Elnagar29). Two tested the effectiveness of water iodisation and whether water is an alternative vehicle for iodisation(Reference Kassim, Moloney and Busili27, Reference Elnagar, Eltom and Karlsson28). The most common nutrition intervention and strategy for treating ID is the Universal Salt Iodisation programme. Table 1 presents a summary of all the included studies in this review.
Table 1. List and details of research articles included in this review
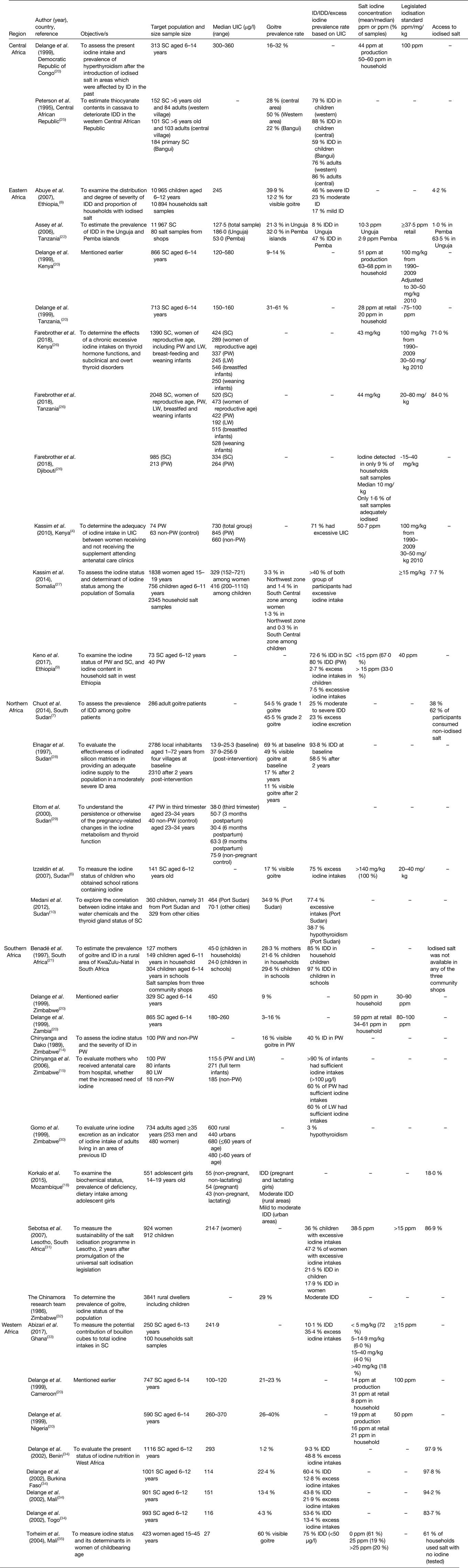
ID, iodine deficiency; IDD, iodine deficiency disorder; LW, lactating women; ppm, part per million; PW, pregnant women; SC, school children; UIC, urinary iodine concentration.
Urinary iodine concentration and goitre in sub-Saharan Africa
From the two Central Africa studies(Reference Delange, De Benoist and Alnwick20, Reference Peterson, Legue and Tylleskär25), the first study reported median UIC showing excessive iodine intakes in both northeast and southeast regions of the Democratic Republic of Congo. However, the prevalence of goitre still ranged between 16 and 32 %(Reference Delange, De Benoist and Alnwick20). In Central African Republic with 437 school children and 187 households, the median UIC indicated mild IDD in the households and severe IDD in the school children. Crude goitre rates of 50 % were observed among the households, including all members older than 6 years. In addition, the goitre prevalence was 28 % among school children in rural and 22 % in urban areas(Reference Peterson, Legue and Tylleskär25).
In Eastern Africa, seven studies were included(Reference Kassim, Ruth and Creeke4, Reference Abuye, Berhane and Akalu8, Reference Keno, Ahrens and Lauvai9, Reference Delange, De Benoist and Alnwick20, Reference Assey, Greiner and Mzee22, Reference Farebrother, Zimmermann and Abdallah26, Reference Kassim, Moloney and Busili27). In Ethiopia, the prevalence of goitre was 39·9 % for children aged 6–12 years in 2007, and 46 and 23 % of the children suffered from severe and moderate IDD, respectively(Reference Abuye, Berhane and Akalu8). IDD seems to be a persistent problem in Ethiopia as Keno and co-authors reported 72·6 % IDD in school children aged 6–12 years and 80 % IDD in pregnant women in 2017(Reference Keno, Ahrens and Lauvai9). Goitre was observed in 21·3 % and 32·0 % of school children in the Unguja and Pemba islands, respectively(Reference Assey, Greiner and Mzee22). In 1999, the goitre prevalence ranged from 9 to 14 % among school children aged 6–14 years in Kenya and 31–61 % in Tanzania(Reference Delange, De Benoist and Alnwick20). This seems to have been addressed as a recent study reported excessive iodine intakes in school children and pregnant women in Kenya(Reference Kassim, Ruth and Creeke4, Reference Farebrother, Zimmermann and Abdallah26), Tanzania and Djibouti(Reference Farebrother, Zimmermann and Abdallah26). Excessive intakes were also reported for school children and women (>40 %) in Somalia(Reference Elnagar, Eltom and Karlsson28), school children in Ethiopia (7·5 %)(Reference Keno, Ahrens and Lauvai9), rural dwellers and Somali refugees (71 %) in Kenya(Reference Kassim, Ruth and Creeke4).
Four studies conducted in Northern Africa were implemented in Sudan(Reference Izzeldin, Crawford and Jooste6, Reference Medani, Elnour and Saeed10, Reference Elnagar, Eltom and Karlsson28, Reference Eltom, Eltom and Elnagar29) and one in South Sudan(Reference Chuot, Galukande and Ibingira7). All the studies reported excess and/or inadequate status of iodine(Reference Izzeldin, Crawford and Jooste6, Reference Chuot, Galukande and Ibingira7, Reference Medani, Elnour and Saeed10, Reference Elnagar, Eltom and Karlsson28, Reference Eltom, Eltom and Elnagar29). In one study, excess iodine intake was observed among 77·4 % participants in Port Sudan (n 31 school children)(Reference Medani, Elnour and Saeed10). Furthermore, a higher prevalence of excess iodine levels (75 %) was also observed in a similar study conducted in 2006 (n 141 children)(Reference Izzeldin, Crawford and Jooste6). The authors emphasised that lack of regulation and monitoring of salt fortification could be the possible reason for excess iodine status among the study population(Reference Izzeldin, Crawford and Jooste6). A study in Western Sudan reported the efficiency of water sources containing iodine-saturated silicon matrices for providing adequate iodine supply to an iodine deficient population (n 2786). The prevalence of goitre decreased from 69 % (baseline) to 17 % (post-intervention) after a 2-year implementation(Reference Elnagar, Eltom and Karlsson28). In a study conducted in South Sudan among patients with endemic goitre (n 286), 54·5 % of the respondents had grade 1 goitre and 45·5 % grade 2 goitre. Goitre was more prevalent among women(Reference Chuot, Galukande and Ibingira7). Furthermore, in another study, it was found that pregnant women (n 50) had significantly lower UIC levels than non-pregnant women(Reference Eltom, Eltom and Elnagar29).
From Southern African countries, eight published papers were included in this review(Reference Chinyanga14, Reference Chinyanga, Chidede and Machisvo15, Reference Korkalo, Freese and Alfthan18, Reference Delange, De Benoist and Alnwick20, Reference Benade, Oelofse and van Stuijvenberg21, Reference Gomo, Allain and Matenga30–Reference Team32). A study undertaken in 924 (including sixty-four pregnant) women and 912 children in Lesotho showed median UIC indicating normal iodine intakes. However, 36 % of the children and 47·2 % of the women had excessive UIC. The study also showed that 21·5 % of the children and 17·9 % of the women had UIC lower than 100 µg/l(Reference Sebotsa, Dannhauser and Jooste31). In South Africa, a cross-sectional survey among 127 mothers with 149 children aged 6–11 years as well as 304 primary school children (6–14 years old) in rural Kwazulu-Natal showed a goitre prevalence of 28·3 % among mothers and 21·6 % among their children. The school children had a goitre prevalence of 29·6 %. Based on the UIC levels, 85 % of the household members and 97 % of the school children had IDD(Reference Benade, Oelofse and van Stuijvenberg21). In many other studies, IDD still existed in the same populations, with visible goitre, especially among adolescent girls in Mozambique(Reference Korkalo, Freese and Alfthan18), pregnant women in Zimbabwe(Reference Chinyanga14) and children in Zimbabwe(Reference Team32), and Zambia(Reference Delange, De Benoist and Alnwick20).
In the Western Africa region, four studies reported excessive intake of iodine(Reference Delange, De Benoist and Alnwick20, Reference Abizari, Dold and Kupka33–Reference Torheim, Granli and Sidibé35). A study was conducted among 250 Ghanaian school children and reported that about a third (35·4 %) of the children had high UIC compared with only 10·1 % with insufficient iodine intakes(Reference Abizari, Dold and Kupka33). In Mali, a study among 423 women showed that 35 % of the women had severe and 40 % moderate IDD. Only 6 % of the women had adequate iodine intakes. While 60 % of the women had visible goitre, only 9 % were classified as without goitre (Table 1)(Reference Torheim, Granli and Sidibé35). Among 590 Nigerian children, the median UIC levels were high. However, the prevalence of goitre was 26 and 40 % in the two areas respectively despite the high UIC levels(Reference Delange, De Benoist and Alnwick20). In addition, the goitre prevalence among children (n 747) was 20 and 23 % in two areas respectively in Cameroon(Reference Delange, De Benoist and Alnwick20).
Multi-country study
In total three studies were conducted in multiple countries(Reference Delange, De Benoist and Alnwick20, Reference Farebrother, Zimmermann and Abdallah26, Reference Delange, Kibambe and Ouedraogo34). A study was conducted in three countries in East Africa by Farebrother and co-authors, and recruited a total of 4636 participants from these three countries. Excess iodine intakes were found among the participants from Kenya and Tanzania(Reference Farebrother, Zimmermann and Abdallah26). In a multi-centre study by Delange and co-authors in four countries in West Africa, namely Benin, Burkina Faso, Mali and Togo, the highest rate of IDD (32·4 %) was found in Burkina Faso and excess iodine intakes (48·8 %) in Benin. The average median UIC was within normal range in four countries; however, still one-third of the participants had UIC levels below the acceptable range. The authors remarked on improvement in iodine status among the population; however, improved monitoring is needed in order to ensure quality of iodine concentration in salt(Reference Delange, Kibambe and Ouedraogo34). Fig. 2 represents that two studies in Central Africa(Reference Delange, De Benoist and Alnwick20, Reference Peterson, Legue and Tylleskär25), five studies in Eastern Africa(Reference Abuye, Berhane and Akalu8, Reference Keno, Ahrens and Lauvai9, Reference Delange, De Benoist and Alnwick20, Reference Assey, Greiner and Mzee22, Reference Kassim, Moloney and Busili27), four studies in Northern Africa(Reference Chuot, Galukande and Ibingira7, Reference Elnagar, Eltom and Karlsson28, Reference Izzeldin, Crawford and Jooste6, Reference Medani, Elnour and Saeed10), six studies in Southern Africa(Reference Chinyanga14, Reference Korkalo, Freese and Alfthan18, Reference Delange, De Benoist and Alnwick20, Reference Benade, Oelofse and van Stuijvenberg21, Reference Sebotsa, Dannhauser and Jooste31, Reference Team32) and four studies in Western Africa(Reference Delange, De Benoist and Alnwick20, Reference Abizari, Dold and Kupka33–Reference Torheim, Granli and Sidibé35) found ID. However, iodine excess was also reported in all regions, except for Central Africa. Although more studies reported IDD, excess iodine intakes should be noted and given attention in future studies.

Fig. 2. (Colour online) Number of studies found iodine deficiency and iodine excess in Africa sub-regions. CA, Central Africa; EA, Eastern Africa; NA, Northern Africa; SA, Southern Africa; WA, Western Africa.
Iodine deficiency disorders interventions and iodine from food sources in sub-Saharan Africa
Salt iodisation is the most effective strategy for addressing IDD globally and also in Africa. However, the success of the iodisation fortification programmes is highly dependent on household coverage(Reference Abizari, Dold and Kupka33). Compulsory salt iodisation was introduced in most of the SSA countries as early as the 1970s in Kenya(Reference Farebrother, Zimmermann and Abdallah26), and most other countries during the 1990s, for example Tanzania(Reference Farebrother, Zimmermann and Abdallah26), Mali(Reference Torheim, Granli and Sidibé35), Zimbabwe(Reference Chinyanga14, Reference Gomo, Allain and Matenga30), South Africa(Reference Benade, Oelofse and van Stuijvenberg21), Ethiopia(Reference Keno, Ahrens and Lauvai9), Lesotho(Reference Sebotsa, Dannhauser and Jooste31), Sudan(Reference Medani, Elnour and Saeed10) and Ghana(Reference Torheim, Granli and Sidibé35). Some countries also obtained iodised salt from their neighbours, for example Djibouti from Kenya(Reference Farebrother, Zimmermann and Abdallah26). The WHO recommended level is 20–40 and 15–40 mg iodine per kg salt respectively, at the production level and household level(19). Not all the studies reported the iodine concentration and access to iodised salt. In addition, many countries in SSA have their own legislation guidelines. In one study from Sudan, 100 % of the salt samples showed high iodine levels of >140 mg/kg, three times as high as the legislated amount(Reference Izzeldin, Crawford and Jooste6). From the results it is clear that most of the salt samples that were analysed had low iodine levels compared with the individual country's legislated amounts, except for Lesotho(Reference Sebotsa, Dannhauser and Jooste31), Zimbabwe(Reference Delange, De Benoist and Alnwick20), Tanzania(Reference Farebrother, Zimmermann and Abdallah26) and Kenya(Reference Kassim, Ruth and Creeke4, Reference Farebrother, Zimmermann and Abdallah26) that had optimum salt iodine levels. Interestingly, the same countries showed higher household access of iodised salt compared with those with low salt iodine levels. In the multi-country study from West Africa, high access levels of iodised salt, ranging from 83·7 % in Togo to 97·9 % in Benin, were also reported(Reference Delange, Kibambe and Ouedraogo34). The goitre patients in three South Sudanese counties (n 286) were mostly the rural poor and all reported sorghum and maize as their predominant food. Only 38 % reported consuming iodised salt(Reference Chuot, Galukande and Ibingira7). Some neighbouring countries in the region, such as Kenya, have over 90 % of their populations consuming iodised salt(Reference Farebrother, Zimmermann and Abdallah26). Chuot and co-authors also showed that despite the consumption of iodised salt, some individuals were still suffering from IDD. Contrary to this, only 50 % of the respondents that consumed non-iodised salt were iodine deficient. The reason for this apparent discrepancy may be that IDD is influenced by differences in diet(Reference Chuot, Galukande and Ibingira7).
Many food sources of iodine exist in SSA. Many food processors use iodised salt in foods, oils and blended cereal products. However, in these products, iodine levels are largely unmonitored(Reference Izzeldin, Crawford and Jooste6). In Ghana, it was estimated that two-thirds of the dietary iodine intake was from bouillon cubes manufactured with iodised salt(Reference Abizari, Dold and Kupka33). Other sources of iodine include excess fluoride levels in drinking-water(Reference Medani, Elnour and Saeed10), water iodination using silicon matrices(Reference Elnagar, Eltom and Karlsson28), supplementation and intake of fortified food(Reference Kassim, Ruth and Creeke4), high consumption of dairy products(Reference Abizari, Dold and Kupka33) and groundwater(Reference Farebrother, Zimmermann and Abdallah26). Water is an essential nutrient consumed by all on a daily basis and is thus a good alternative for iodine supplementation. In fact, silicon matrices containing sodium iodide in well water sources were used in a study in Sudan in 1997(Reference Elnagar, Eltom and Karlsson28). Plants, such as cassava as well as low iodine content in the soil of Central Africa, have been known to impact IDD(Reference Peterson, Legue and Tylleskär25). In Ethiopia, cassava consumption may have contributed to the regional variation in the IDD prevalence(Reference Abuye, Berhane and Akalu8). Cassava, a staple in Central and West Africa, is widely consumed in some parts of the country. It contains a goitrogenic substance known as cyanogenic glucoside, which upon conversion to thiocyanate in the body exacerbates ID when iodine intake is marginal or low(Reference Abuye, Berhane and Akalu8, Reference Peterson, Legue and Tylleskär25).
Iodine-testing kits and titration may be used to test household salt iodine levels. In a multi-centre study in four African countries, iodine-testing kits overestimated the absence and the elevated content of iodine. As a result, the percentage of salt samples meeting the legal requirements of at least 50 ppm was about two-thirds (64·3 %) according to the kits but only about one-third (36·3 %) according to titration. Therefore, titration seems to be a more accurate method. Based on titration, iodine was undetectable in 0·5 % of the samples; its content was 1–14 ppm in 17·3 %, 15–49 ppm in 37·4 %, 50–100 ppm in 36·3 % and above 100 ppm in the last 8·5 %, including 0·5 % in which it was above 200 ppm. The access to iodised salt at the household level in the four countries varied from 83·7 to 97·8 %, with a global value of 94·3. It was above 90 % in three of them(Reference Delange, Kibambe and Ouedraogo34).
Although many studies from most countries still reported the prevalence of IDD and goitre, excessive iodine intakes have been observed in some countries, namely Somali refugee women in Kenya (71·5 %)(Reference Kassim, Ruth and Creeke4), Sudan (>33 %)(Reference Izzeldin, Crawford and Jooste6), Ghana (35·4 %)(Reference Abizari, Dold and Kupka33), Somalia (40 %)(Reference Kassim, Moloney and Busili27), Benin (48·8 %)(Reference Delange, Kibambe and Ouedraogo34), Mali (21·9 %)(Reference Delange, Kibambe and Ouedraogo34), Togo (13·4 %)(Reference Delange, Kibambe and Ouedraogo34) and Burkina Faso (12·8 %)(Reference Delange, Kibambe and Ouedraogo34). Research indicates that children and women who are pregnant and lactating are the most at risk populations for IDD because of an increase in iodine excretion during pregnancy(Reference Sebotsa, Dannhauser and Jooste31). Thus, pregnant and lactating women are required to have their iodine levels monitored to prevent complications of ID(Reference Chinyanga, Chidede and Machisvo15). WHO, UNICEF and International Council for Control of Iodine Deficiency Disorders published a supplementation recommendation statement that pregnant and lactating women should consume 250 mg iodine daily(23). Many refugee camps in Africa, including the one in Kenya where the women had excessive intakes, adopted this statement and rely on international organisations to provide food items for these refugees. While the iodine amounts of these donated food items are unknown, they are expected to provide 150–200 mg iodine per 8786·4 kJ daily. No adverse health consequences have been reported among Somali refugees in Kenya, but the health concerns of taking excessive iodine among refugees in Africa still remains(Reference Kassim, Ruth and Creeke4).
Conclusions
Salt iodisation has been recognised as a sustainable and cost-effective universal strategy to combat IDD(Reference Bryce, Coitinho and Darnton-Hill36). In low-resource countries the implementation of salt iodisation may cause significant changes in the marketing and distribution channels. Elnagar and co-authors predicted that it might take years to achieve universal fortification and reduction of IDD(Reference Elnagar, Eltom and Karlsson28). In Africa, almost 33 % of the total population was affected by ID in 1990. In the most severely affected African countries, IDD has improved through household utilisation of iodised salt, iodised oil and iodised water(Reference Kassim, Ruth and Creeke4, Reference Peterson, Legue and Tylleskär25, Reference Delange, Kibambe and Ouedraogo34). Earlier studies reported more IDD and higher prevalence rates of goitre whereas the more recent studies (2010 and later) reported excessive intakes and a lower prevalence of goitre(Reference Kassim, Ruth and Creeke4, Reference Chuot, Galukande and Ibingira7, Reference Keno, Ahrens and Lauvai9, Reference Medani, Elnour and Saeed10, Reference Korkalo, Freese and Alfthan18, Reference Farebrother, Zimmermann and Abdallah26, Reference Kassim, Moloney and Busili27, Reference Abizari, Dold and Kupka33). In most of the countries it appears that the iodised salt fortification has made a considerable impact on the prevalence of IDD and goitre, but it is clear that none of the WHO indicators for achieving sustainable elimination of IDD have been fulfilled in SSA. There are also some countries that still have a high prevalence of IDD. In Ethiopia, for example, 72·3 % of the children and 80 % of pregnant women are still suffering from IDD(Reference Keno, Ahrens and Lauvai9). Similarly in Mozambique, a recent study has shown IDD in adolescent girls(Reference Korkalo, Freese and Alfthan18). Therefore, improvements in access to iodised salt and awareness of its importance are needed with a special focus on the rural population in these countries(Reference Korkalo, Freese and Alfthan18).
Furthermore, in most countries, the salt iodine levels were lower than the legislated standards and household access to iodised salt was also low. This indicates that other food and water sources are likely contributing to the excessive intakes observed in the more recent studies(Reference Chuot, Galukande and Ibingira7, Reference Keno, Ahrens and Lauvai9, Reference Farebrother, Zimmermann and Abdallah26, Reference Abizari, Dold and Kupka33). Although the results indicate that IDD is still a concern in some SSA countries, the emerging problem of excess iodine intakes should be a warning for policy makers and programme implementers to critically consider iodine fortification levels, and not to implement blanket additional supplementation to avoid hyperthyroidism without regular routine iodine assessments. Excessive iodine intakes may have adverse health impacts on the thyroid gland greater than those induced by iodine deficient diets. There is thus an urgent need to conduct further population-based studies to investigate iodine intakes of different population groups. It is important to provide a fortification/supplementation strategy to meet the optimal dietary iodine supplemental needs of each country. Also, effective efficacy and safety monitoring is needed to prevent under or over iodisation at the production level, and iodine losses during distribution, and storage of salt in order to reach adequate iodine levels in salt for household use. Moreover, in IDD populations, awareness programmes should be implemented to raise awareness of the importance of using iodised salt at the household level. Variations of the geology and iodine-rich food resources in the different SSA countries are evident, requiring that programme implementers take this into account as well as existing iodine-related interventions to address IDD effectively without exposing the regularly targeted women and children to excess iodine intake levels.
Acknowledgements
The authors would like to thank Professor Abulkadir Egal (Centre of Sustainable Livelihood, Vaal University of Technology, South Africa) for reviewing and providing advice on this paper.
Financial Support
None.
Conflict of Interest
None.
Authorship
W. O-T. and B. A. Z. A. were responsible for the conception the review topic. S. S., B. A. Z. A., Y. Z-N., U. M., M. M., L-L. P. and W. O-T. took part in the data search, review of included papers and drafting of this review paper. S. S. coordinated the review process. All authors reviewed and approved the final version.





