Iron deficiency is a major global public health problem, despite extensive investment in interventions for its prevention and treatment. Iron deficiency is the most common micronutrient deficiency worldwide, with the greatest burden in women of reproductive age and young children(1, Reference Kassebaum and Collaborators2). Iron is essential for brain development, myelination, growth and cognitive function(Reference Lonnerdal3). Inadequate iron status has been associated with adverse health outcomes, including deficits in cognitive function (i.e. concentration, short-term memory, reaction time)(Reference Nelson4, Reference Stivelman5), as well as reduced physical work capacity and endurance(Reference Haas and Brownlie6, Reference McClung and Murray-Kolb7).
Biofortification is a promising and sustainable agriculture-based intervention with the potential to improve nutritional status worldwide, particularly in vulnerable populations(Reference Bouis, Hotz and McClafferty8). It differs from conventional fortification approaches in that it aims to increase micronutrient levels in staple crops during plant growth, rather than through manual means during grain processing. The method involves the targeted breeding of staple food crops to increase their intrinsic micronutrient content(Reference Bouis, Hotz and McClafferty8, Reference Prentice, Mendoza and Pereira9). This approach allows for leveraging existing markets and delivery systems while vulnerable populations are not required to change consumption behaviours in order to receive more diverse, nutritious diets. Increasing the micronutrient content of staple foods items (that constitute the main portion of the diet) through biofortification can be beneficial even if the increase in micronutrient content is small(Reference Prentice, Mendoza and Pereira9). In this review, biofortification refers to the process by which the vitamin and mineral content of staple crops is increased through agronomic practices, conventional plant breeding or modern biotechnology(Reference Bouis, Hotz and McClafferty8).
Previous research demonstrated that iron biofortification interventions were efficacious in improving iron biomarkers(Reference Finkelstein, Haas and Mehta10), and a recent review concluded that iron-biofortified staple crops are efficacious in improving iron status and further highlighted the need to assess functional outcomes(Reference Finkelstein, Haas and Mehta10). However, to date, no systematic reviews have been conducted to examine the efficacy of biofortification interventions on health outcomes.
This review was conducted to examine the efficacy of iron-biofortified staple food crop interventions on improving iron status and functional outcomes, including cognitive performance and physical performance. We conducted meta-analyses to combine findings from included randomised trials to inform public health programmes and to incorporate biofortification as a strategy to target iron deficiency in at-risk populations.
Methods
Types of studies
Controlled trials (i.e. randomised, quasi-randomised), with randomisation at the individual or cluster level, were eligible for inclusion in this review. Research studies that had only been published in abstract form were considered for inclusion if sufficient information was provided to determine eligibility, study design and quality.
Types of participants
We included studies of participants from the general population (including pregnant or lactating women), without respect to participant sex, age, nationality or race. We excluded studies of interventions targeted towards participants with critical illnesses or severe co-morbidities.
Types of interventions
We included studies that examined the efficacy of iron-biofortified staple crops on health outcomes. Interventions providing iron-biofortified staple crops that were not GM (non-GMO), in comparison to conventional crops, were considered without any restrictions on population characteristics or country of location. Only interventions with a duration of at least 28 d were considered.
Types of outcome measures
The primary outcomes examined in this review are presented in Table 2.
Iron status
Primary functional outcomes were: (1) anaemia, defined as Hb concentrations below 120 g/l, in accordance with WHO criteria, adjusted for smoking and altitude, where applicable; and (2) iron deficiency, defined as serum ferritin (SF) <15·0 μg/l in primary analyses; and as total body iron (TBI; mg/kg), as calculated by Cook's Equation(Reference Cook, Flowers and Skikne11), <0·0 mg/kg and soluble transferrin receptor >8·3 mg/l in additional analyses.
Functional outcomes
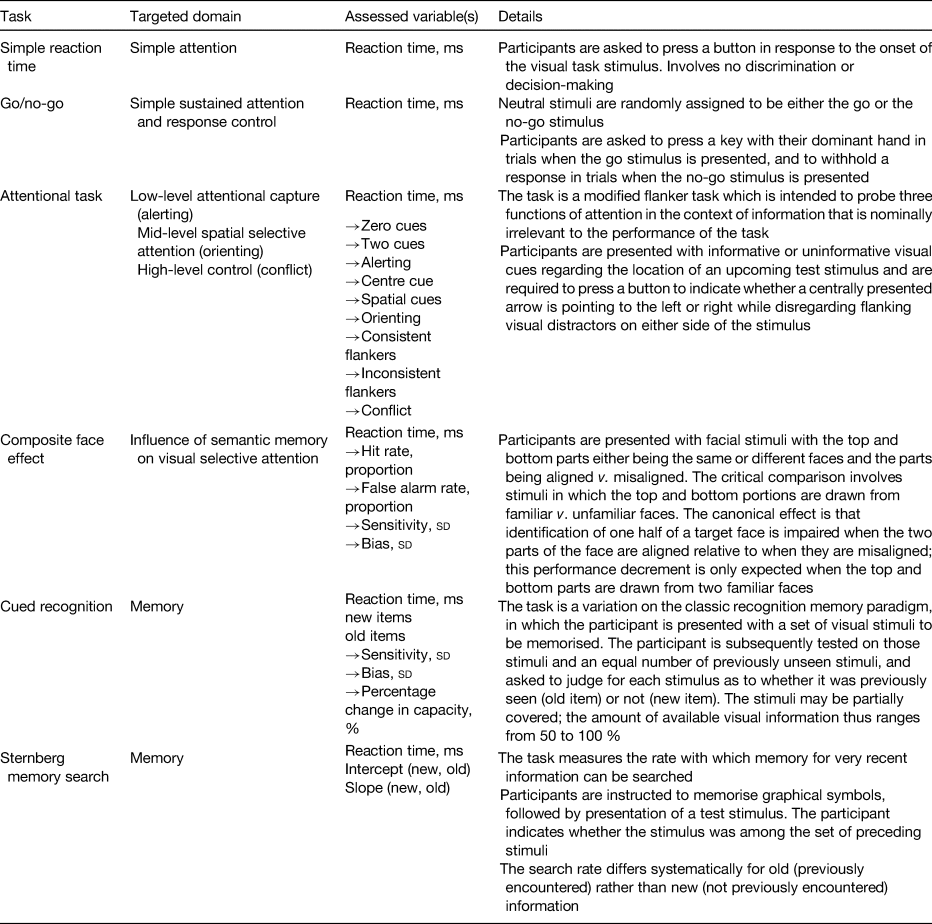
Primary functional outcomes were: (1) cognitive function, as defined by the study authors (e.g. formal tests addressing reaction times and accuracy of responses in tasks targeting attention, memory and other cognitive domains); (2) physical performance, as defined by the study authors (e.g. output produced per work hour, such as wages earned when dependent on production output); and (3) other functional outcomes, as defined by the trial authors (e.g. education/academic achievement, emotional health, psychomotor development).
Other outcomes
Any adverse effects (as defined by study authors) were considered as secondary outcomes.
Search methods for identification of studies
We conducted a structured literature search with the use of MEDLINE electronic databases. Relevant Medical Subject Headings (MeSH) terms were used to identify published studies on 2 August 2018, with no language or date restrictions. The MeSH terms used are summarised in Table 1, and the search strategy PRISMA is summarised in Fig. 1. Additional sources were identified from bibliographies of published studies and from manual searches of related articles in references. An additional search was conducted to find review articles, which were examined to cross-reference other relevant studies. Search results were screened by two independent reviewers (A. F., L. S. H.) to determine if studies met the inclusion criteria.
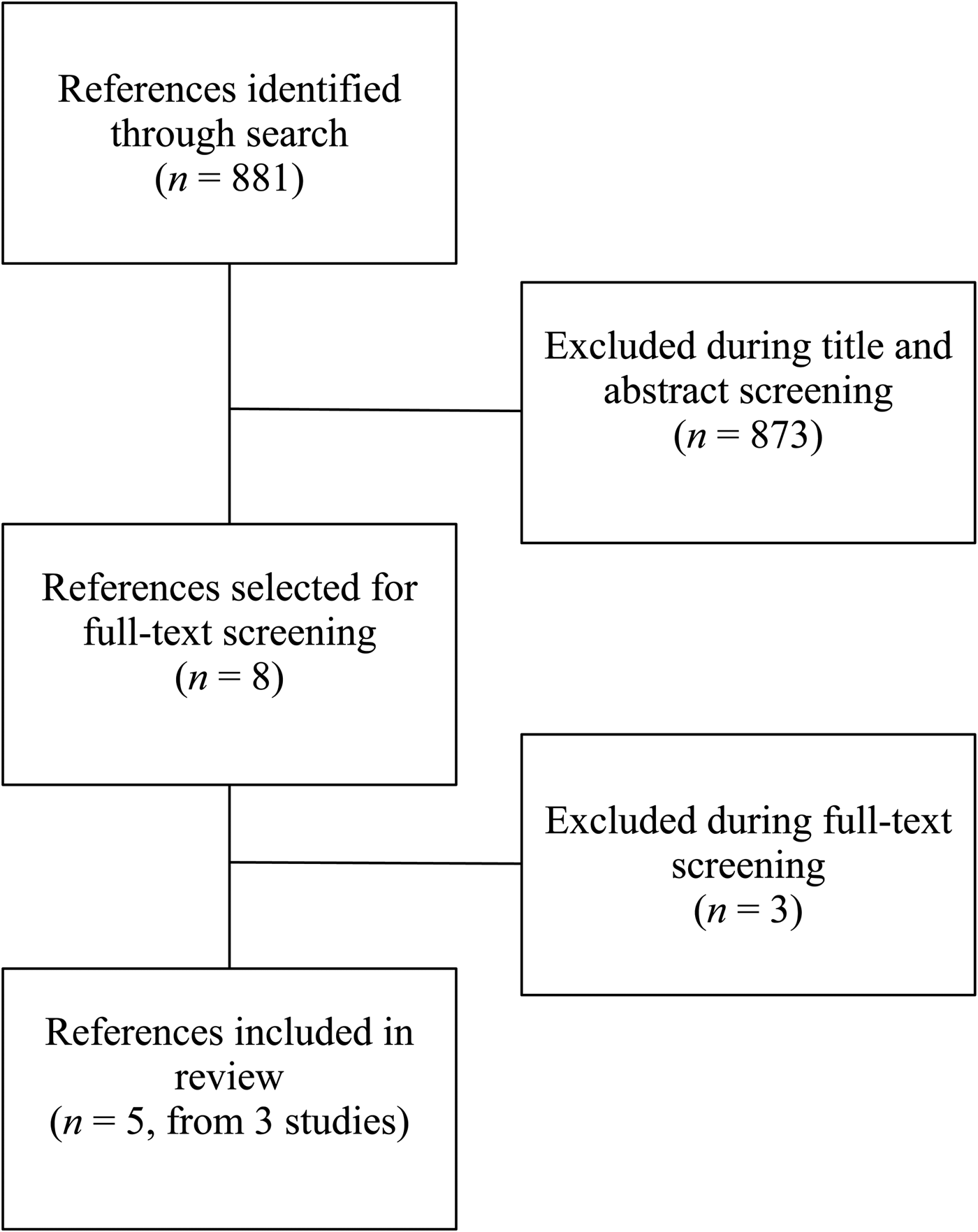
Fig. 1. PRISMA flow diagram.
Table 1. MEDLINE search strategy
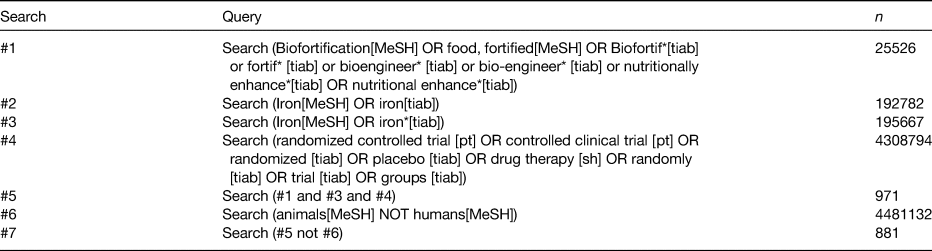
MeSH, Medical Subject Headings.
The search was conducted on 2 August 2018.
Table 2. Primary outcomes for iron status and functional parameters

SF, serum ferritin; sTfR, soluble transferrin receptor; TBI total body iron.
* TBI = –(log10 (sTfR (mg/l) × 1000/SF (μg/l)−2·8229)/0·1207) (Cook's Equation)(Reference Cook, Flowers and Skikne11).
Data collection and analysis
Selection of studies
A standardised form for data extraction was developed, piloted and used to ensure accurate data extraction from included studies. Data from studies identified as potentially eligible upon screening were extracted independently by two authors (A. F., L. S. H.). All discrepancies were resolved through discussion and consultation with an additional review author (J. L. F. or S. M.). Extracted information included: study characteristics (i.e. year of study, duration of intervention, kind of iron-biofortified foods, setting, inclusion and exclusion criteria, recruitment strategy, sample size, rates of attrition), population characteristics (e.g. sex, age, occupation, socio-economic status) and all outcomes reported (e.g. iron status, anthropometric, cognitive function, physical performance, any adverse effects and any other outcomes reported by the study authors). We contacted study authors to request any data that were either missing or required additional clarifications.
Risk of bias and quality assessment
Risk of bias was independently assessed by two authors (A. F., L. S. H.), using criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions. The protocol was registered in PROSPERO (CRD42018118329), the international prospective register of systematic reviews of the University of York and the National Institute for Health Research.
For each randomised trial, potential sources of bias were examined, including selection, performance, detection, attrition, reporting and other potential biases. Methods used for random sequence generation and allocation concealment were examined for potential selection biases; methods for blinding of study participants and personnel were examined for performance biases; methods for blinding outcome assessors were examined for detection bias; completeness of data and study attrition were examined for potential biases; study protocols and methods sections were compared with reported results to examine for potential reporting bias; and any other concerns identified that could potentially introduce bias were identified. For each study, each of the afore-mentioned areas was evaluated and classified into low, high or unclear risk of bias.
Data synthesis
Statistical analyses were conducted in the Cochrane Review Manager software (RevMan v5.3 2014), and meta-analyses were conducted using random-effects models (DerSimonian and Laird method). Weights used in meta-analyses are reported in each figure.
Subgroup analyses
Where data were available, we planned to conduct the following subgroup analyses for primary outcomes: baseline anaemia (e.g. Hb < 120 v. ≥120 g/l) and baseline iron deficiency (e.g. SF < 15·0 v. ≥15·0 µg/l; TBI < 0·0 v. ≥0·0 mg/kg) status.
Results
This review summarises findings from randomised efficacy trials investigating the effects of iron-biofortified staple food crops on iron status, cognitive performance and work performance. Our structured search identified 881 abstracts, of which 873 were excluded during initial abstract screening (i.e. insufficient intervention length, interventions without biofortified crops, not randomised control trials). Eight publications (from four studies) underwent full-text screening; three publications (from two studies) were excluded due to the use of non-biofortified foods. A total of five publications from three randomised efficacy trials were included in this review; the PRISMA flow diagram is presented in Fig. 1.
Randomised efficacy trials
Three randomised efficacy trials assessing the performance of iron-biofortified staple food crops on iron status have been conducted and their results published to date. The staple food crops used in those trials were rice, beans and pearl millet. Two of the studies measured cognitive performance in a subset of participants via behavioural tasks assessing memory and attention (Tables 2 and 3).
Table 3. Characteristics of randomised efficacy feeding trials of iron-biofortified crops
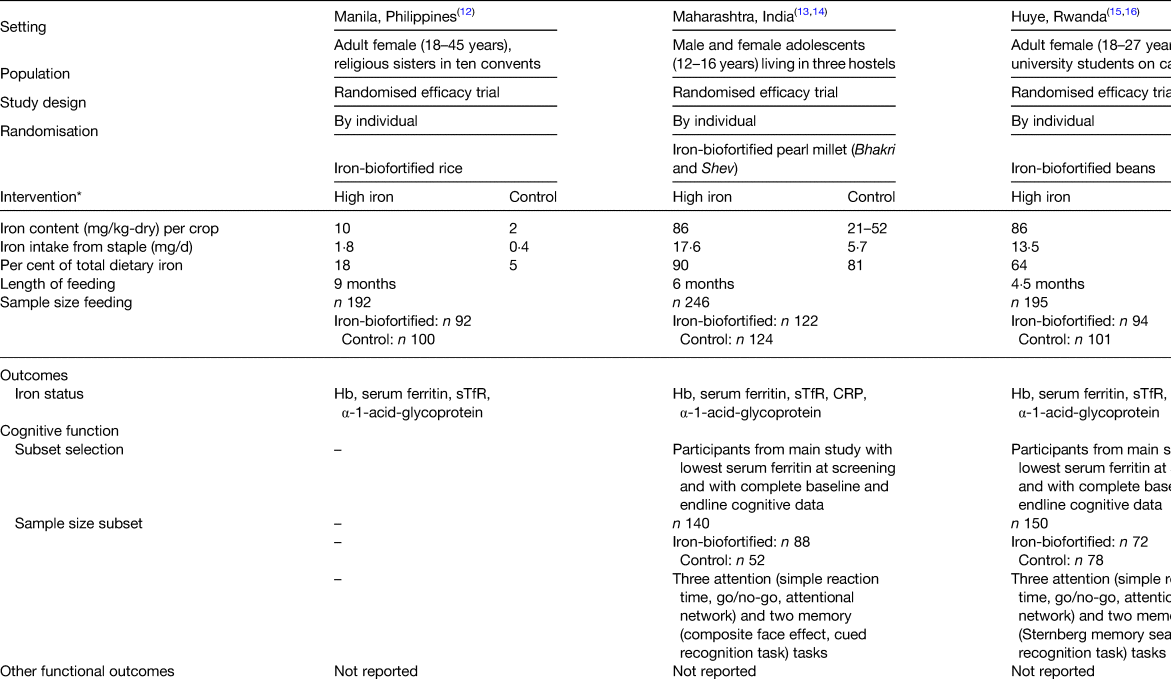
AGP, α-1-acid-glycoprotein; CRP, C-reactive protein; SF, serum ferritin; sTfR, soluble transferrin receptor.
* Modified from(Reference Boy, Petry and Cercamondi27).
Iron-biofortified rice consumption in religious sisters in the Philippines
A double-blind randomised controlled trial (RCT) was conducted to examine the efficacy of iron-biofortified rice (Oryza sativa) consumption on parameters of iron status in 192 religious sisters living in ten convents around metro Manila, Philippines(Reference Haas, Beard and Murray-Kolb12). Parameters of work performance or cognitive function were not assessed in this study or related sub-studies. Participants were randomly assigned to daily ad libitum consumption of iron-biofortified rice (3·21 mg/kg Fe, n 92) or a local variety of conventional rice (0·57 mg/kg Fe, n 100) for 9 months. This resulted in a 17 % difference in total iron consumed in the iron-biofortified group compared with the control group throughout the intervention period. Iron status (Hb, SF, soluble transferrin receptor, TBI, α-1-acid glycoprotein) was measured at base- and endline (9 months). Iron status. At baseline, 28 % of participants were anaemic (Hb < 120 g/l) and 34 % were iron deficient (SF < 15·0 µg/l). In analyses among non-anaemic participants, iron-biofortified rice consumption increased SF (P = 0·02) concentrations and TBI (P = 0·05) during the trial, representing a 20 % increase after controlling for baseline values and daily rice consumption.
Iron-biofortified pearl millet consumption in adolescents in India
A double-blind RCT examined the efficacy of iron-biofortified pearl millet (Pennisetum glaucum) consumption among 246 adolescents (age 12–16 years) in Maharashtra, India (NCT02152150)(Reference Finkelstein, Mehta and Udipi13). Adolescents daily consumed 200–300 g of either iron-biofortified (86 mg/kg Fe, n 122) or conventional (21–52 mg/kg Fe, n 124) pearl millet in the form of Bhakri flatbread during the 6-month follow-up. Iron status (Hb, SF, soluble transferrin receptor, TBI, C-reactive protein, α-1-acid glycoprotein) was measured at base-, mid- (4 months) and endline (6 months). Iron status. At baseline, 28 % of adolescents were anaemic (Hb < 120 g/l) and 43 % were iron deficient (SF < 15·0 µg/l). Iron-biofortified pearl millet significantly increased SF concentrations and TBI levels after 4 months compared with conventional pearl millet. The effects of iron-biofortified pearl millet on iron status were also greater among adolescents who were iron deficient at baseline, compared with those who were not iron deficient. Cognitive outcomes. In a subset of 140 study participants (n 88 in biofortification group, n 52 in control group), with the lowest ranked SF concentrations, measures of cognitive function were evaluated at base- and endline (6 months)(Reference Scott, Murray-Kolb and Wenger14). The measures consisted of attention (i.e. simple reaction time, go/no-go, attentional network) and memory (i.e. composite face effect, cued recognition) tasks (Table 4). In the cognitive subset, 33 % of participants were anaemic and 50 % were iron deficient at baseline. The group consuming iron-biofortified pearl millet demonstrated greater improvement in cognitive measures of both attention and memory, compared with the group consuming conventional pearl millet. Specifically, the iron-biofortification intervention significantly improved all three measures of the attentional network task, i.e. alerting, orienting and conflict, compared with the conventional pearl millet group, which also showed a decline in performance in orienting and conflict. Furthermore, participants in the iron-biofortification group showed significant improvements in the cued recognition task (suggesting an improved ability to adapt to increasing workload), compared with the conventional group. Overall, findings from these analyses indicate a benefit of consuming iron-biofortified over conventional pearl millet on measures of attention and memory.
Table 4. Cognitive assessment instruments and respective memory and attention domains
Iron-biofortified bean consumption in iron-depleted Rwandan women of reproductive age
A double-blind RCT was conducted to determine the efficacy of iron-biofortified bean (Phaseolus vulgaris) consumption among 195 iron-depleted (SF < 20·0 µg/l) female university students (18–27 years) in Huye, Rwanda (NCT01594359)(Reference Haas, Luna and Lung'aho15). Participants received either iron-biofortified (86 mg/kg Fe, n 94) or conventional (50 mg/kg Fe, n 101) beans twice daily for 128 d (i.e. 4·5 months). Iron status (Hb, SF, soluble transferrin receptor, TBI, C-reactive protein, α-1-acid glycoprotein) was measured at base-, mid- (random serial sample) and endline (4·5 months). Iron status. At baseline, 37 % of women were anaemic (Hb < 120 g/l) and 86 % were iron deficient (SF < 15·0 µg/l). The iron-biofortified bean intervention significantly increased Hb concentrations by 3·0 g/l (from 121 to 124 g/l), SF concentrations by 5·5 µg/l (from 10·0 to 15·4 µg/l) and TBI by 1·5 mg/kg (from −0·7 to 0·8 mg/kg). In contrast, in the conventional intervention group, Hb concentrations decreased by 1·2 g/l (123–122 g/l), SF concentrations increased by 3·7 g/l (from 10·0 to 13·6 g/l) and TBI increased by 1·0 mg/kg (from −0·7 to 0·3 mg/kg). Cognitive outcomes. In a subset of 150 study participants (n 72 from the biofortification group, n 78 from the control group) with the lowest SF concentrations at baseline, measures of cognitive function were evaluated at base- and endline (4·5 months)(Reference Murray-Kolb, Wenger and Scott16). In the cognitive assessment sub-study, a total of 43 % of participants were anaemic and 92 % were iron deficient at baseline. Cognitive outcomes assessed were attention (i.e. simple reaction time, go/no-go, attentional network) and memory (i.e. mnemonic performance: Sternberg memory search, cued recognition). Iron-biofortified bean consumption predicted a 17 % larger change in the reaction time for the selective spatial attention and improved efficiency and specificity of both memory retrieval and memory search, compared with consumption of conventional beans.
Meta-analyses
Based on the evidence generated by these randomised trials, we conducted meta-analyses to examine the efficacy of iron-biofortification interventions on iron status and functional outcomes. We used a meta-analytical approach to estimate a summary measure for the potential benefit of consuming different iron-biofortified staple crops, with the aim to inform future efficacy trials and effectiveness studies.
Risk of bias in included studies
Risk of bias was assessed for all included studies in the meta-analysis and is presented in Fig. 2. In the three main randomised trials included, risk of bias was classified as low for most criteria. Participants were randomly assigned to interventions, but the sequence generation for randomisation was not described in any of the trials. Both participants and field personnel were blinded to the intervention in all three randomised trials, but blinding procedures used in RCT conducted in India(Reference Finkelstein, Mehta and Udipi13) and the Philippines(Reference Haas, Beard and Murray-Kolb12) were not clearly described. Furthermore, in the Philippines trial, it was not clear if all outcomes were reported as specified, as the study protocol and trial registration were not available. In the two sub-studies focusing on cognitive performance, participants were selected based on their iron biomarkers at baseline (e.g. iron deficiency or SF < 15·0 µg/l), indicating a high selection bias. Furthermore, the two intervention groups in Scott et al. (Reference Scott, Murray-Kolb and Wenger14) demonstrated baseline differences in iron status parameters indicating a high risk of other bias.

Fig. 2. Risk of bias assessment for all included studies. Risk of bias was assessed by two authors independently and classified as either low (+), high (−) or unclear (?) for each respective domain. (A) Random sequence generation (selection bias); (B) allocation concealment (selection bias); (C) blinding of participants and personnel (performance bias); (D) blinding of outcome assessment (detection bias); (E) incomplete outcome data (attrition bias); (F) selective reporting (reporting bias); (G) other bias. Numbers in parentheses indicate listed references.
All three of the RCT reported iron status outcomes, two studies reported on cognitive outcomes in the domains of attention and memory, and none of the studies reported on physical performance or other functional outcomes.
Effects of interventions on iron status
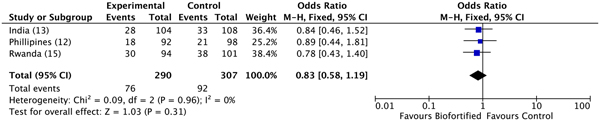
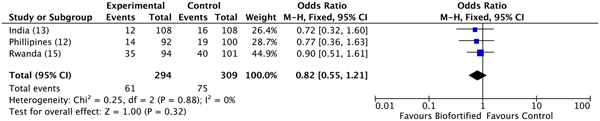
A previous review reported results for Hb, SF and TBI concentrations(Reference Finkelstein, Haas and Mehta10). Findings demonstrated improvements in SF concentrations and TBI concentrations (but not Hb), with additional potential to benefit in individuals who were iron deficient at baseline(Reference Finkelstein, Haas and Mehta10). Anaemia. We conducted meta-analyses of data from the three included randomised trials, examining the efficacy of iron-biofortified interventions on anaemia and iron deficiency at endline. The effects of iron-biofortified staple crops on anaemia (Hb < 120 g/l) are presented in Fig. 3. There were no significant effects of iron-biofortified interventions on anaemia at endline (anaemia, OR 0·83, 95 % CI 0·58, 1·19). Iron deficiency. The effects of iron-biofortified staple crops on iron deficiency defined based on SF and TBI are presented in Fig. 4 (SF < 15·0 µg/l) and Fig. 5 (TBI < 0 mg/kg), respectively. There were no significant effects of iron-biofortified interventions on iron deficiency at endline (SF < 15·0, OR 0·86, 95 % CI 0·61, 1·23; TBI < 0 mg/kg, OR 0·82, 95 % CI 0·55, 1·21).
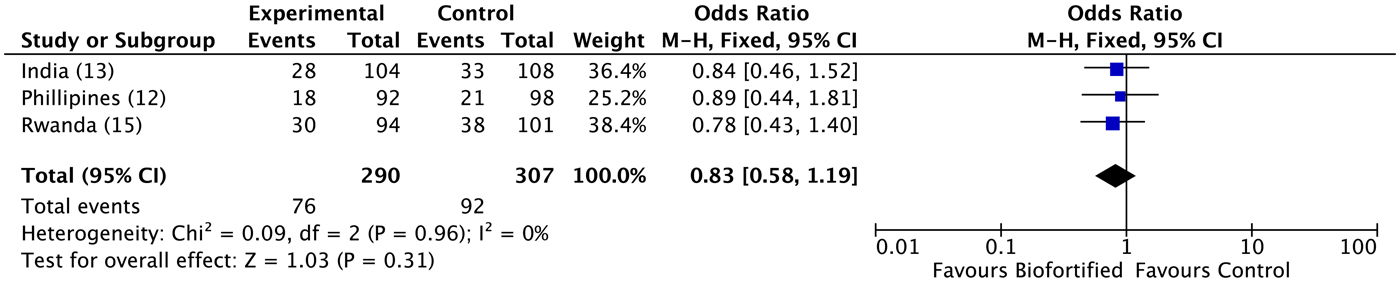
Fig. 3. Effect of iron-biofortified crop interventions on anaemia (Hb < 120 g/l). Numbers in parentheses in the study/subgroup column indicate listed references.
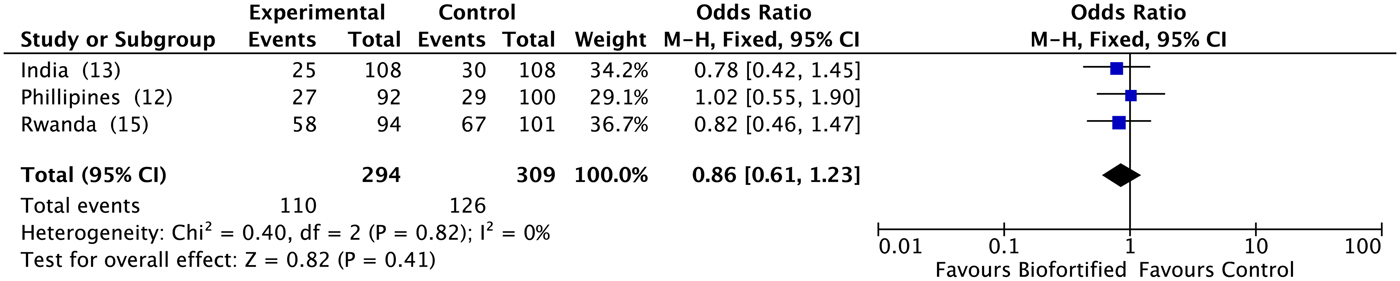
Fig. 4. Effect of iron-biofortified crop interventions on iron deficiency (serum ferritin < 15·0 µg/l). Numbers in parentheses in the study/subgroup column indicate listed references.
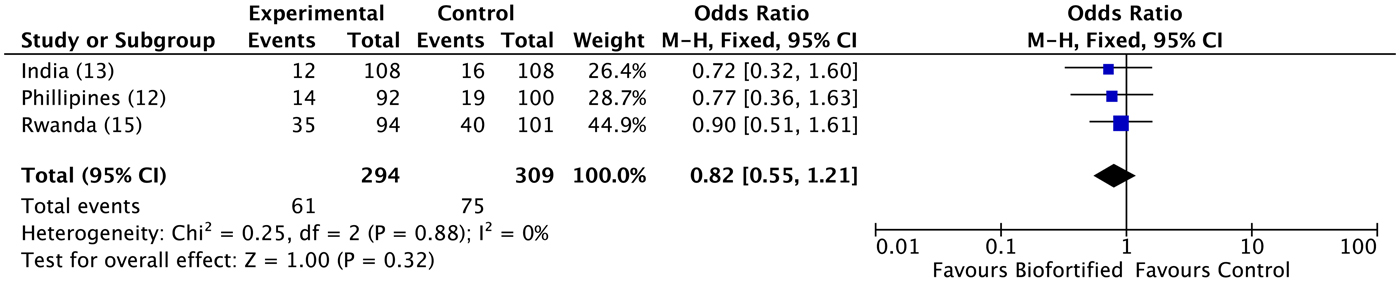
Fig. 5. Effect of iron-biofortified crop interventions on iron deficiency (total body iron < 0·0 mg/kg). Numbers in parentheses in the study/subgroup column indicate listed references.
Effects of interventions on cognitive function
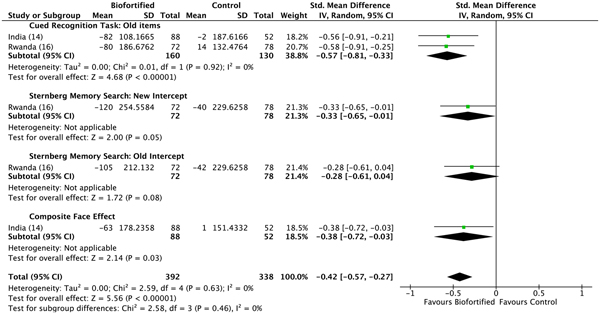
We conducted meta-analyses of data from the included RCT, examining the efficacy of iron-biofortified interventions on measures of cognitive function, attention and memory. Attention. The effects of iron-biofortified staple crops on cognitive measures of attention are presented in Fig. 6. A significant improvement in performance (as indicated by a reduction in reaction times) in the iron-biofortified v. the conventional groups was found in the go/no-go task (reaction time −0·25, 95 % CI −0·48, −0·01) and the following of attentional tasks: two cues (reaction time −0·25, 95 % CI −0·49, −0·02); alerting (reaction time −0·33, 95 % CI −0·67, 0·00); spatial cues (reaction time −0·35, 95 % CI −0·61, −0·10); orienting (reaction time −0·37, 95 % CI −0·61, −0·13). Iron-biofortified crop interventions significantly improved overall performance in tasks assessing the cognitive domain attention (reaction time −0·22; 95 % CI −0·32, −0·12) (Fig. 6). Memory. The effects of iron-biofortified staple crops on cognitive measures of memory are presented in Fig. 7. Participants in the iron-biofortification groups demonstrated significantly reduced reaction times in the cued recognition task (reaction time −0·57, 95 % CI −0·81, −0·33); the Sternberg memory search with new items (reaction time −0·33, 95 % CI −0·65, −0·01); and the composite face effect (reaction time −0·38, 95 % CI −0·72, −0·03). Iron-biofortified crop interventions significantly improved overall performance in tasks assessing the cognitive domain memory (reaction time −0·42, 95 % CI −0·57, −0·27).
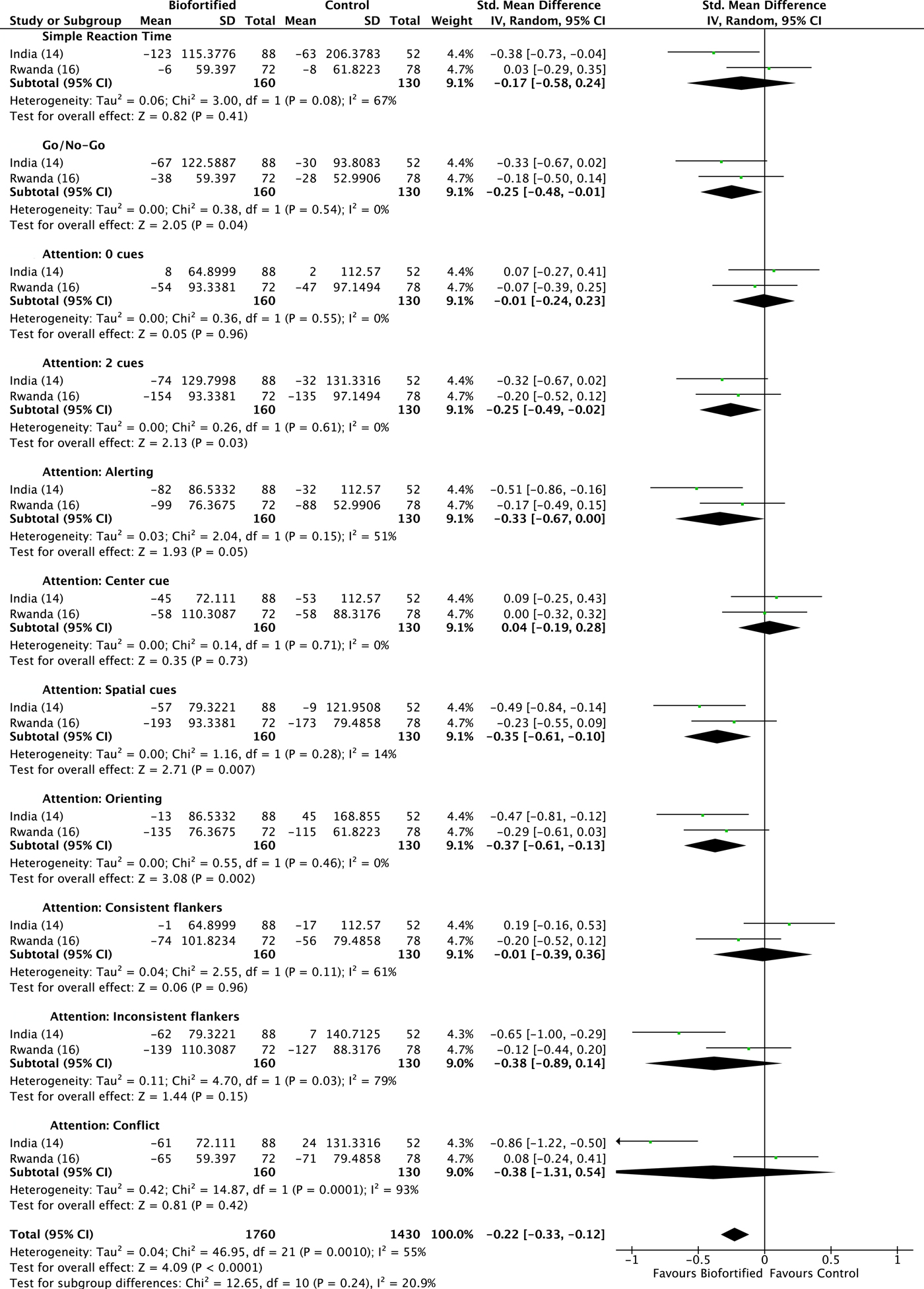
Fig. 6. Effect of iron-biofortified crops on cognitive outcomes: attention domain. Performance was measured as difference in reaction times. Details on the procedures of the performed tests can be found in Table 4. Numbers in parentheses in the study/subgroup column indicate listed references.
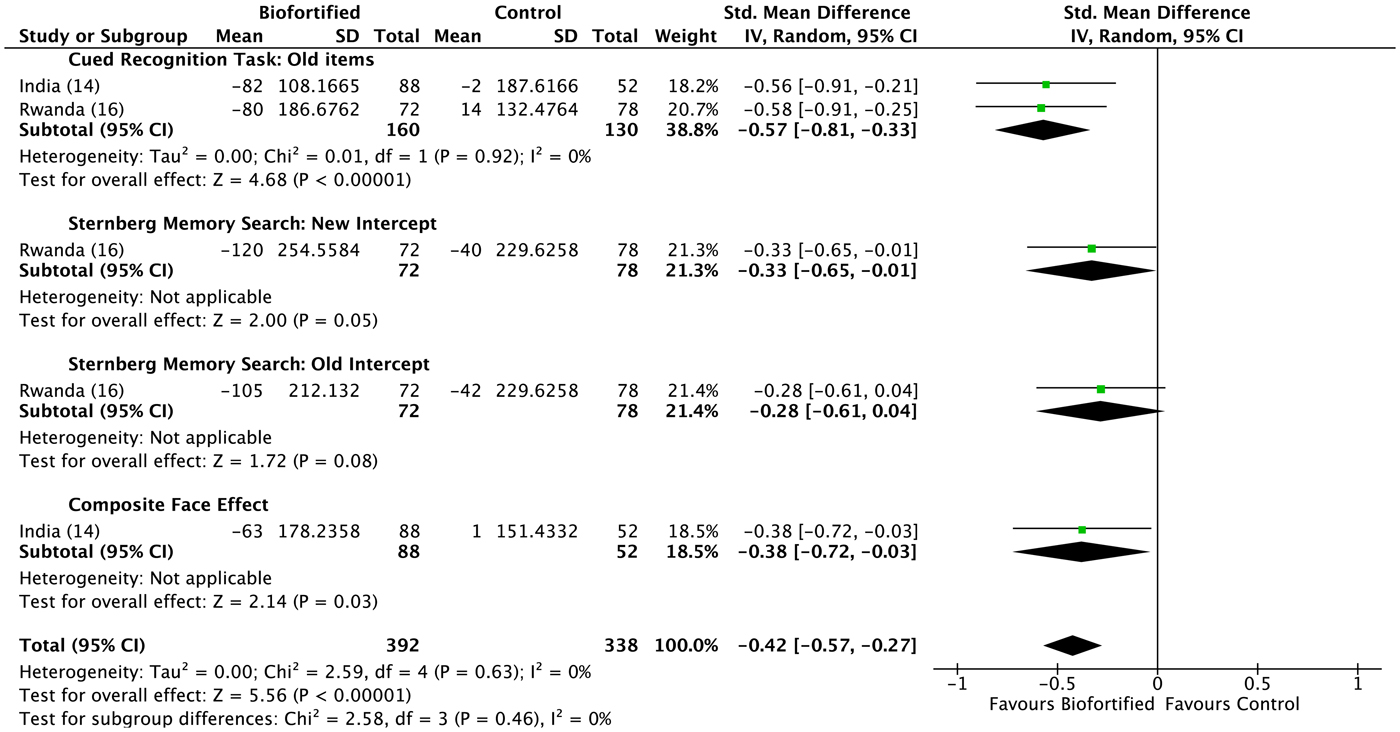
Fig. 7. Effect of iron-biofortified crops on cognitive outcomes: memory domain. Performance was measured as difference in reaction times. Details on the procedures of the performed tests can be found in Table 4. Numbers in parentheses in the study/subgroup column indicate listed references.
Discussion
The findings from these meta-analyses highlight the potential of iron-biofortification interventions to improve cognitive performance with respect to attention and memory domains in vulnerable populations. This may inform the development of future trials and effectiveness studies of the potential impact of iron-biofortified crops on iron status and functional outcomes.
There is limited evidence on the potential efficacy of biofortified crop interventions on functional outcomes, such as cognition and physical performance. Iron is essential for normal brain development and cognitive function including, but not limited to energy metabolism, neurotransmitter production, and myelination(Reference Sandstead17, Reference Georgieff18). Several studies, most frequently in rats, have shown that changes in the brain occur in iron-deprived states, and that these changes are associated with deficits in cognitive development(Reference Beard19, Reference Felt and Lozoff20). Accordingly, cognitive impairment is among the most important functional consequences of iron deficiency(Reference McCann and Ames21). Iron-deficient infants and children have delayed attention, poor recognition memory, are more likely to be withdrawn from social interactions, and have long-term cognitive deficits. Emerging evidence from longitudinal studies suggest that uncorrected iron deficiency in infancy is associated with persistent cognitive deficits into early childhood, highlighting the importance of correcting these deficits in the critical early years of life(Reference Lozoff, Beard and Connor22, Reference Lozoff and Georgieff23).
Iron trials to examine the effects of iron repletion on cognitive function in human subjects and animals have provided conflicting evidence, and are constrained by limitations in study designs. However, human and animal studies with stronger study designs for causal inference suggest that iron repletion improves cognitive function(Reference Lubach and Coe24, Reference Idjradinata and Pollitt25).
Findings from this analysis suggest that iron biofortification interventions improved cognitive performance with respect to attention and memory domains. No research to date has been published on the efficacy of iron biofortification on physical performance or other functional outcomes. However, research in this area is forthcoming: the RCT in Rwanda (NCT01594359)(Reference Haas, Luna and Lung'aho15) and Maharashtra, India (NCT02152150)(Reference Finkelstein, Mehta and Udipi13) included evaluation of physical performance as a secondary outcome measure, and a recently completed RCT in young children in Mumbai, India (NCT02233764)(Reference Mehta, Finkelstein and Venkatramanan26) included cognition, immune function and growth as outcomes. Previous studies in different populations, including iron supplementation, have demonstrated that iron interventions improved physical work capacity and endurance(Reference Haas and Brownlie6, Reference McClung and Murray-Kolb7), and provide biological plausibility of a potential benefit of iron biofortification on physical performance outcomes. Further research is needed to determine the efficacy of biofortified crop interventions on other functional outcomes and in different high-risk populations.
Findings expand upon previous research on the efficacy of iron-biofortification interventions demonstrating significant improvements in continuous measures of iron status such as SF concentrations; however, in these analyses, no significant effect was observed on categorical measures such as iron deficiency. This may be inherent to the statistical analysis where the power is sufficient to detect significant differences in continuous but not in categorical outcomes; alternatively, the effect size may be too small to move the population distribution adequately to affect anaemia and iron deficiency.
Comparing the effects of biofortification with those of fortification and supplementation should be considered when planning public health programmes and interventions. Although we are not aware of any studies comparing biofortification against conventional fortification or supplementation, meta-analyses of interventions focused on fortification or supplementation suggest larger effect sizes for these approaches. However, it is still unclear whether delivering the extra dose of the nutrients through the food matrix may be beneficial and this was partly the objective behind incorporating functional outcomes in the discussed trials and the meta-analyses. Future public health programmes and interventions should be designed to take advantage of the complementarity of these approaches; for example, in a setting with a high burden of iron deficiency, supplementation may be the preferred short-term intensive approach with fortification or biofortification more of a sustainable long-term maintenance strategy.
This review has several limitations, which warrant caution in the interpretation of findings. For example, only baseline and endline data were included in our analyses, and study durations differed between all studies; studies had heterogeneous designs, including duration, frequency of feeding administration, and included different biofortified crop interventions, risk populations and settings. The diversity of the populations, settings and design of the randomised trials constrains the comparability of findings. This is particularly true for the cognitive performance tests, as the studied populations differed regarding their age and sex (female and male adolescents v. female adults), setting (India v. Rwanda) and educational level (university students v. adolescents attending boarding schools). None of the reported studies assessed the long-term impact of iron-biofortified staple crop administration in children, where changes in the developing haematological and functional parameters may be more pronounced. More studies assessing developmental aspects are needed. Tasks should be standardised between different studies, while still accounting for potential cultural biases in the studied populations. Additional domains of cognitive performance should be considered for testing.
Findings to date from randomised trials suggest that iron-biofortified crops are an efficacious intervention to improve continuous measures of iron status. Furthermore, findings from this systematic review indicate that the consumption of iron-biofortified crops can improve cognitive function, in terms of attention and memory. Future studies are needed to generate the required evidence to successfully scale up biofortification efforts for populations in need. Assessment of other functional outcomes and in other high-risk populations is warranted to inform the development and scale-up of biofortified interventions to improve human health.
Financial Support
None.
Conflict of Interest
J. L. F., J. D. H. and S. M. have received competitive grant funding for conducting randomised efficacy trials of biofortified crops from HarvestPlus. J. D. H. has served as an expert consultant for HarvestPlus. S. M. also has an equity interest in a diagnostic start-up, planning to commercialise his work on point-of-care methods of nutritional assessment.
Authorship
J. L. F., A. F., and L. S. L. wrote the first draft of the manuscript; A. F. and L. S. L. extracted the data in duplicate and conducted data analyses in RevMan; J. L. F., J. D. H., and S. M. provided guidance in the interpretation of findings from data analyses; all authors revised the manuscript and reviewed the final version; J. L. F. has responsibility for final content.