- BMD
bone mineral density
- GRF
ground-reaction forces
- MES
minimum effective strain
- RCT
randomised controlled trials
Osteoporosis is a condition of skeletal fragility whereby depleted bone mass and compromised bone structure weaken bone to such an extent that fractures occur from minimal trauma. The disease is a major public health problem with great social and economic importance. One in five men and one in two women in the UK >50 years of age will suffer an osteoporosis-related fracture in their lifetime(Reference van Staa, Dennison, Leufkens and Cooper1) and the annual cost for all fractures is £1·5×109(Reference Torgerson and Dolan2).
Two mechanisms that principally determine adult bone health are peak bone mineral density (BMD) at skeletal maturity and the rate of bone loss with advancing age; thus, maximising premenopausal BMD is a critical strategy for the prevention of osteoporosis and resultant fractures later in life. A substantial body of literature has established that participating in regular physical activity can positively improve bone mineral status(Reference Borer3, Reference Kohrt, Bloomfield, Little, Nelson and Yingling4). The advantage of exercise over methods such as dietary intervention is that it increases the skeleton's resistance to fracture by improving and maintaining both BMD and neuromuscular competency, thus reducing both skeletal fragility and predisposition to falls(Reference Kohrt, Bloomfield, Little, Nelson and Yingling4).
Exercise recommendations for cardiovascular fitness have already been precisely defined(Reference Pollock, Gaesser and Butcher5), but it is unlikely that the same exercise prescription applies to cardiovascular health and skeletal health, and to both pre- and post-menopausal women. Bone's response to exercise differs across the lifespan according to the age and health of the individual(Reference Beck and Snow6), yet in the past research has focused predominantly on post-menopausal women and studies involving premenopausal women are sparser.
The aim of the present review is to describe what is known about exercise as an intervention to increase BMD among premenopausal women and, learning from past studies, how future interventions could potentially fill the gaps in current knowledge.
Determinants of bone strength
There is a dynamic regulatory system in bone that adapts its strength to its mechanical environment by alterations in the amount and orientation of bone at each skeletal site. This adaptation process is described as Wolff's Law after the German scientist who first recognised in 1892 how ‘every change in environment is followed by change in internal architecture’, and how there is a mechanism for functional adaptation in the skeleton. Since then, the principle has further developed into the mechanostat theory(Reference Frost7), which describes the mechanism of bone adaptation like a thermostat (Fig. 1). Bone has various set points of minimum effective strain (MES) that are independently and interdependently determined by local (e.g. previous load-bearing), systemic (e.g. hormones) and external (e.g. diet) factors, as well as age and genetics(Reference Skerry8). If mechanical loading increases and exceeds the relative MES (i.e. what bone is accustomed to), bone formation:bone resorption is temporarily unbalanced until mineral mass increases and structural adaptation occurs so that bone becomes strong enough to support the greater functional demands being placed on it. A new MES is then established. Conversely, if loading on bone decreases and falls below the MES threshold there is a rapid loss of BMD, because a less-demanding external environment allows the metabolic cost of maintaining mineral to be reduced(Reference Lanyon9). This loss is substantial, as decreases in BMD of approximately 1%/month have been reported during times of immobilisation or bed rest(Reference Shackelford, LeBlanc and Driscoll10) and weightlessness(Reference LeBlanc, Schneider and Shackelford11), compared with the average loss of <1%/year normally observed in older post-menopausal women.

Fig. 1. Diagrammatic representation of predicted change in bone mass relative to applied strain according to the mechanostat theory. MES, minimum effective strain; –, loss; +, gain. (Adapted from Frost(Reference Frost7).)
The mechanical competence of bone is a function not only of its intrinsic material properties (mass, density and stiffness), but also of its structural properties (size, shape and geometry). Dual-energy X-ray absorptiometry is the method most commonly used to measure areal BMD (g/cm2) and bone mineral content (g) because of its speed, precision, low radiation exposure and availability of reference data(Reference Watts12). However, the understanding of bone's response to mechanical loading is restricted by these two-dimensional skeletal outcomes that represent only one part of overall bone strength. The development of hip structural analysis algorithms has enabled estimation from dual-energy X-ray absorptiometric scans of certain bone structural changes that may occur along with bone densitometric changes to further increase bone strength(Reference Kaptoge, Dalzell and Jakes13, Reference Petit, McKay, MacKelvie, Heinonen, Khan and Beck14). Studies have shown that exercise can positively alter structural variables, sometimes in the absence of detectable changes in BMD(Reference Adami, Gatti, Braga, Bianchini and Rossini15, Reference Järvinen, Kannus and Sievanen16).
Animal studies
Studies using animal models have established the characteristics of mechanical loading for optimal bone formation. First, it is clear that strain from mechanical loading needs to be of a high magnitude. As explained previously by the mechanostat theory, strain needs to exceed the set MES, which is usually about 2000–3000 microstrain. After this point and until the upper MES threshold, at which excessive loading causes damage to bone, there is a graded dose–response relationship between the peak strain magnitude and the change in the mass of bone tissue present(Reference Lanyon17, Reference Rubin and Lanyon18). Second, a high rate of strain provides a greater osteogenic stimulus than the same peak strain achieved more slowly. Rat ulnae subjected to high-strain-rate (0·1 με/s) in vivo loading have been demonstrated to have a 54% greater osteogenic response than a moderate-strain-rate group (0·03 με/s), who in turn show a 13% larger response than a low-strain-rate group (0·018 με/s)(Reference Mosley and Lanyon19). Third, bone adaptation is driven by unusual strain distributions. The ‘error strain distribution hypothesis’(Reference Lanyon9) proposes that bone cells enhance the skeleton's structural competence by adjusting to perceived deviations from normal in the distribution of dynamic strains. For example, strain produced by loading in torsion may be less osteogenic than the same strain produced by loading in longitudinal compression. Furthermore, it has been suggested that the distribution of strain may be more important than its magnitude(Reference Turner and Robling20), as unusual patterns of strain can stimulate an osteogenic response at a lower MES(Reference Lanyon17). Fourth, the number of cycles of loading applied to bones appears to become unimportant once a certain threshold level has been reached(Reference Rubin and Lanyon18). As few as thirty-six loading cycles lasting only 72 s have been shown to stimulate maximal bone formation in turkey ulnae(Reference Lanyon17) and 100 jumps per d produce the same bone response in rats as forty jumps per d(Reference Umemura, Ishiko, Yamauchi, Kurono and Mashiko21). Likewise, it has been found that the rate of bone formation in avian ulnae when compared with that of contralateral bones is not significantly elevated by five consecutive days of 100 low-magnitude-loading cycles, but is elevated by separating the 100 cycles into ten bouts with 10 s rest between bouts(Reference Srinivasan, Weimer, Agans, Bain and Gross22). Thus, prolonged periods of mechanical loading saturate the bone's adaptive response to mechanical load. Following from this finding, it has been demonstrated that inserting regular rest periods between loading bouts maintains the mechanosensitivity of bone, whereas static load applied continuously produces no effect different from disuse(Reference LaMothe and Zernicke23). In rats allowed time to recover between thirty-six identical daily loading cycles, 14 s of recovery resulted in significantly higher (66–190%) relative bone formation rates when compared with three shorter recovery periods (0·5, 3·5 and 7 s); furthermore, in the longer term, 4 h rest doubles bone formation whilst a further 4 h of recovery restores full mechanosensitivity to desensitised bone cells(Reference Robling, Burr and Turner24). Thus, mechanical loading is not only more osteogenic when short rest intervals are inserted between cycles, but also when each bout of loading is separated by several hours. However, the optimum frequency of loading bouts, i.e. the number of times per week that is required for maximising bone accrual, has not yet been established.
Finally, animal studies have demonstrated that bone has an age-dependent response to mechanical loading and the importance of oestrogen for bone's adaptation to be fully realised has been highlighted. Following an 8-week period of unilateral daily loading of a physiologically-normal level of strain, bone cross-sectional area in 1-year-old turkey ulnae has been found to increase by 30·2% as compared with their intact contralateral control ulnae, whereas the areal properties of the 3-year-old turkeys remain essentially unchanged (–3·3%)(Reference Rubin, Bain and McLeod25). The authors have suggested that a physical signal that is clearly osteogenic in the young adult skeleton is scarcely acknowledged in older bone tissue. Whether this difference in response represents a deterioration of the ability of older bone cells to perceive these physical signals or a failure of their capacity to respond is not yet clear. It may also be linked to the role of oestrogen in amplifying the osteogenic response to a single period of loading, because in animal models of post-menopausal osteoporosis exercise prevents early bone loss after ovariectomy but does not actually stimulate bone formation(Reference Flieger, Karachalios, Khaldi, Raptou and Lyritis26, Reference Peng, Tuukkanen and Vaananen27).
The advantage of animal studies is that they can be very tightly controlled and in vivo adaptation of bone mineral content and strength can be assessed, although it cannot be confirmed that human bone responds in exactly the same way as animal bone since invasive procedures cannot be carried out on human subjects. Consequently, clarification of the effects of mechanical loading on bone in human subjects relies on cross-sectional observations of different human populations and longitudinal intervention studies.
Cross-sectional studies
Evidence for the importance of mechanical loading comes from numerous studies that have compared the BMD values of individuals who take part in different types of sports. Differences in BMD have consistently been shown between athletes participating in sports involving high ground-reaction forces (GRF) and those participating in low- or no-impact sports(Reference Heinonen, Kannus, Sievanen, Haapasalo, Manttari and Vuori28–Reference Pettersson, Nordstrom, Alfredson, Henriksson-Larsen and Lorentzon30). The largest differences have regularly been observed in gymnasts whose hip and spine BMD values are 30–40% higher than those of long-distance runners(Reference Robinson, Snow-Harter, Taaffe, Gillis, Shaw and Marcus31); a plausible explanation being the greater magnitude of impact forces generated in gymnastics movements (10–12×body weight)(Reference McNitt-Gray32) compared with running (3–5×body weight)(Reference Duncan, Blimkie, Cowell, Burke, Briody and Howman-Giles33). Moreover, not only are high-impact sports associated with a greater BMD, but also athletes involved in high-impact sports have a greater section modulus (a predictor of strength in bending)(Reference Nikander, Sievanen, Heinonen and Kannus29) (Fig. 2). On the other hand, the bone properties of swimmers, who do not experience any loading, are no different from those of sedentary controls(Reference Heinonen, Kannus, Sievanen, Haapasalo, Manttari and Vuori28).
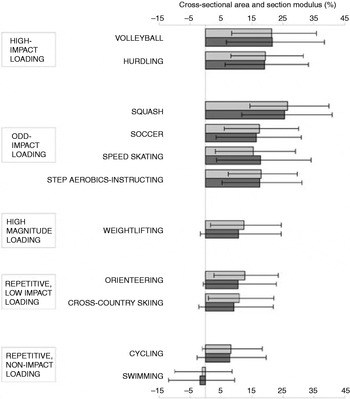
Fig. 2. Differences in cross-sectional area (![]() ) and section modulus (a predictor of strength in bending;
) and section modulus (a predictor of strength in bending; ![]() ) between athletes participating in sports of different loading modalities and controls. Values are means and 95% CI represented by horizontal bars. Where the 95% CI does not cross the zero line (the value for the controls) the difference was significant (P<0·05). (From Nikander et al.(Reference Nikander, Sievanen, Heinonen and Kannus29) Reproduced from J Bone Miner Res (2005) 20, 520–528 with permission of the American Society for Bone and Mineral Research.)
) between athletes participating in sports of different loading modalities and controls. Values are means and 95% CI represented by horizontal bars. Where the 95% CI does not cross the zero line (the value for the controls) the difference was significant (P<0·05). (From Nikander et al.(Reference Nikander, Sievanen, Heinonen and Kannus29) Reproduced from J Bone Miner Res (2005) 20, 520–528 with permission of the American Society for Bone and Mineral Research.)
Related to the concept of strain distribution, sports such as squash, volleyball and gymnastics, which stress bone in a variety of directions, are associated with a higher BMD than sports consisting of only one direction of movement, e.g. running(Reference Heinonen, Kannus, Sievanen, Haapasalo, Manttari and Vuori28). Similarly, zig-zag hopping produces higher compression, tension and shear strains than those of jogging and walking, and hence it has been proposed that this kind of activity may be an optimal tibial bone-strengthening exercise(Reference Milgrom, Miligram, Simkin, Burr, Ekenman and Finestone34). The low BMD reported amongst long-distance runners(Reference Snow35) supports the finding from animal studies that bone adapts to the current loading magnitude and that increasing the duration of loading above the established MES confers no additional benefit, although the low BMD may be exacerbated by the common occurrence of menstrual irregularities (i.e. oestrogen deficiency) in this particular population(Reference Drinkwater36). Other human studies support the greater importance of loading magnitude compared with its duration. For example, it has been reported that >100 accelerations per d are positively associated with higher BMD providing they exceed 3·9 g(Reference Heikkinen, Vihriala, Vainionpaa, Korpelainen and Jamsa37). Thus, it is likely that human bone would also become less desensitised to loading if regular rest intervals intersperse loading cycles; yet, as with animal studies, the response of human bone to varying weekly frequencies of loading has not been defined. Along with the type, duration and intensity, frequency of exercise is an important aspect of exercise prescription and warrants investigation.
Cross-sectional unilateral studies
The major limitation of cross-sectional studies is self-selection bias, i.e. individuals with a higher BMD may be more predisposed to participate in sports and exercise. Their diets and other lifestyle habits may contribute to their success in maintaining a high BMD and it is likely that they have been participating in regular physical activity since childhood. Indeed, such long-term exercisers seem to have greater BMD than those who started exercising in adulthood(Reference Nelson and Bouxsein38). Cross-sectional studies that compare the limbs within the same individual reduce the confounding effect of selection bias. Significant differences in bone density and structure between the dominant and non-dominant limb are attributed to lifetime loading of the favoured dominant side(Reference Chilibeck, Davison, Sale, Webber and Faulkner39, Reference MacIntyre, Adachi and Webber40). The effect of loading is even more marked when comparing the arms of racquet-sport players. The difference in bone mineral between tennis players' playing arm and non-playing arm has been estimated to be 13%(Reference Kannus, Haapasalo, Sievanen, Oja and Vuori41). The age-related response to loading is also apparent in such studies, as the side-to-side differences in BMD that have been noted in pubescent players compared with age-matched controls are not manifested until the girls reached Tanner Stage III, which is the age associated with the adolescent growth spurt(Reference Hääpasalo, Kannus and Sievanen42). In contrast, players who start playing after 30 years of age when bone has matured do not experience the same geometric adaptation as that seen in young players(Reference Nara-Ashizawa, Liu and Higuchi43).
Longitudinal studies
Although cross-sectional unilateral studies reduce selection bias, they cannot prove that exercise causes an increase in BMD. Moreover, reaching conclusions from observations of different athlete groups is difficult because of baseline differences between individuals in hormone levels and Ca intake etc., differences in prescription of the exercise training between groups and uncertain assessment of the loading modality of different sports.
The strongest evidence comes from longitudinal intervention studies, particularly randomised controlled trials (RCT). Despite being fewer in number and weaker in treatment effect, reported RCT continue to support the contention that mechanical loading has a beneficial effect on the skeleton. Older sedentary women (60–74 years of age) who were randomly allocated to perform a training programme involving impact activities were shown to increase their femoral-neck BMD by 3·5% after 11 months, whilst no significant BMD changes were found in the women who undertook non-impact activities or no training at all(Reference Kohrt, Ehsani and Birge44). The findings of another RCT have emphasised that the peak load is more important than the number of loading cycles in increasing bone mass(Reference Kerr, Morton, Dick and Prince45). In this trial post-menopausal women were assigned to a 1-year-long resistance-training regimen that prescribed either three sets of eight repetitions or three sets of twenty repetitions and it was concluded that high loads and low repetitions increase BMD to a greater extent than endurance programmes of low loads and many repetitions.
RCT have also supported the evidence from animal and cross-sectional studies that the osteogenic response of bone to loading is age dependent. A 9-month step-aerobic programme produced greater bone gain in exercising premenarcheal girls than in sedentary premenarcheal girls, but not in exercising postmenarcheal girls(Reference Heinonen, Sievänen, Kannus, Oja, Pasanen and Vuori46), thereby supporting the hypothesis that young bone has the strongest response to mechanical loading. Nonetheless, it is clear that exercise continues to benefit the skeleton through the other decades of life. The RCT that have examined effects of brief high-impact exercise in premenopausal women have shown positive effects on BMD (Fig. 3), but the capacity of the aged skeleton to adapt to the mechanical stress of exercise seems weaker(Reference Bassey, Rothwell, Littlewood and Pye47), perhaps because of inadequate oestrogen levels and inadequate dietary Ca(Reference Borer3). This observation is consistent with findings from animal models and cross-sectional studies. After the menopause mechanical loading may serve more to prevent bone loss than to produce large increases in bone mineral, as some RCT examining the effects of exercise in post-menopausal women have shown that the training group maintains BMD while the control group continues losing the BMD, as would be expected in this population(Reference Engelke, Kemmler, Lauber, Beeskow, Pintag and Kalender48). Physical activity and oestrogen therapy have been reported to have additive effects on bone in older post-menopausal women(Reference Kohrt, Snead, Slatopolsky and Birge49), although one study has suggested that brief high-intensity resistance exercise alone is as effective as the combination of exercise and hormone-replacement therapy in early post-menopausal women(Reference Maddalozzo, Widrick, Cardinal, Winters-Stone, Hoffman and Snow50). This discrepancy may be explained by the suggestion that loading influences bone through oestrogen receptor α(Reference Lee and Lanyon51), which is down regulated in the later post-menopausal period. Lesser effects of exercise on bone after menopause may also be explained by the tendency to use lower-intensity interventions in this population, in order to reduce the risk of injury or improve compliance.
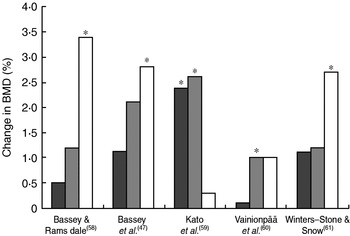
Fig. 3. Summary of bone mineral density (BMD) changes observed in randomised controlled intervention trials involving high-impact exercise in premenopausal women. (![]() ), Lumbar spine; (
), Lumbar spine; (![]() ), femoral neck; (□), trochanter. The increase in the training group was significantly greater than that of the control group: *P<0·05.
), femoral neck; (□), trochanter. The increase in the training group was significantly greater than that of the control group: *P<0·05.
A recent meta-analysis of selected RCT that have investigated the effect of exercise on bone mass has revealed positive effects of exercise on the lumbar spine and femoral neck in premenopausal women(Reference Wallace and Cumming52). Based on yearly estimated changes in BMD, the overall treatment effect was found to be 1·5 (95% CI 0·6, 2·4)% at the spine and 0·7 (95% CI −0·3, −1·7)% at the femoral neck. Although collectively RCT show that regular exercise can delay or halt bone loss in women (Table 1), the exercise-induced increases in BMD reported in longitudinal studies are much smaller and less convincing than the results of cross-sectional studies. As described earlier, the lack of consistency between studies is probably caused by the heterogeneity of the trials. The extent of variation relating to the study populations (e.g. pre- or post-menopausal women), the type, length and intensity of the exercise programmes and the duration of the follow-up periods can make meaningful meta-analysis not feasible(Reference Ernst53). There are a number of limitations to some of the earlier RCT concerning exercise and bone: (1) the exercise programmes were sometimes too general rather than specific in loading the hip or spine, which were the clinically-important sites measured; (2) considering the physiological limits of bone formation and remodelling, the duration of the protocols was sometimes too short to observe significant effects of a lifestyle intervention like exercise; (3) most studies used BMD as their primary outcome measure, which is a suboptimal surrogate for bone fracture rates; (4) many trials had small sample sizes that are associated with type II error.
Table 1. Changes in bone density in exercise relative to control group in published meta-analyses of randomised controlled trials

LS, lumbar spine; FN, femoral neck.
* Martyn-St James & Carroll(Reference Martyn-St James and Carroll66).
† Wallace & Cumming(Reference Wallace and Cumming52).
‡ Wolff et al.(Reference Wolff, van Croonenborg, Kemper, Kostense and Twisk67)
§ Kelley & Kelley(Reference Kelley and Kelley68).
Intervention studies that have investigated the effects of exercise training on BMD cover a range of exercise types, intensities, frequencies and durations, but very few have compared different prescriptions within the same trial, so it is difficult to determine the optimum. This aspect is important because as well as recommending certain types of exercise and how long to exercise for, individuals also need to know how many times per week to exercise. It is clear that there is a need for randomised trials that compare the effects of different exercise prescriptions on bone.
Longitudinal unilateral studies
Although RCT avoid some of the limitations involved in cross-sectional studies, differences in genetic and environmental determinants of BMD between individuals (e.g. Ca intake, baseline activity level, oestrogen level etc.) may still introduce some variance and, along with some of the previously mentioned weaknesses in design, the training programmes used may not produce the maximum osteogenic response in the skeletal sites being measured. In addition, RCT often struggle to recruit large numbers of participants. Unilateral training programmes overcome some of these limitations, as each participant has their own matched control limb, hence it can be confirmed that any differences between the trained and control limb have been caused by the exercise intervention and not any other factor. Furthermore, the paired design reduces the required sample size.
Very few RCT have used a unilateral study design. Unilateral strength training of the upper limb(Reference Heinonen, Sievanen, Kannus, Oja and Vuori54) or lower limb(Reference Vuori, Heinonen, Sievanen, Kannus, Pasanen and Oja55) does not affect BMD; the lack of exercise effect seen in the premenopausal women may be explained by the low compliance rate reported (56% and 78% respectively), loading may have been inadequately different in magnitude and distribution from the habitual loading of the already-active subjects (their Ca intake was <800 mg) and there was no control for menstrual irregularities. On the other hand, a training effect of upper-limb strength training has been observed(Reference Kerr, Morton, Dick and Prince45), which may be explained by the high compliance rate of the participants (82%) and the likelihood that the exercises were a greater stimulus for the ageing bones of the sedentary women. Thus, if the exercise intervention is designed specifically for the population being examined, a unilateral training programme would serve as a useful way to isolate the effects of mechanical loading on bone.
Defining the optimal exercise prescription for bone health
If evidence demonstrates that brief rapid-onset high-intensity unusually-distributed strains produce a maximal osteogenic response, it follows that low-repetition high-impact jumping would be an ideal type of activity to stimulate bone formation. Jumping is an efficient activity because one jump produces two rapid reversals of strain (take-off and landing), and it is also a feasible activity since its short duration (<2 min for fifty jumps) means that it can be conveniently fitted into daily living. Indeed, jumping has been found to produce GRF and muscle actions that provide large stimuli for bone. Jumping modest heights translate into GRF ranging between 2 and 5×body weight(Reference Bassey, Rothwell, Littlewood and Pye47, Reference McKay, Tsang, Heinonen, MacKelvie, Sanderson and Khan56), which as demonstrated with implanted hip prostheses are then almost trebled when converted into in vivo compressive forces on the hip(Reference Bassey, Littlewood and Taylor57). Interventions incorporating ten to 100 jumps performed three to seven times weekly increases BMD in young adult women(Reference Bassey, Rothwell, Littlewood and Pye47,58–Reference Winters-Stone and Snow61) (Fig. 3), demonstrating that this higher-impact loading elicits the overload necessary to stimulate bone formation.
Considering the strengths of a unilateral exercise design and the previously mentioned effectiveness of a high-impact activity like jumping, it follows that unilateral single-legged jumping in the form of hopping exercises may stimulate an even greater bone response. Hypothetically, GRF values would double if only one leg supported the weight of the whole body, as happens during a hop, as opposed to body weight being distributed on two legs, as is the case during a jump. An important feature of jumping is that it involves strain distributions on the skeleton that are atypical for women who do not engage in high-impact sports(Reference Bassey, Rothwell, Littlewood and Pye47), thereby placing unusual but effective mechanical strain on bones. Accordingly, multidirectional hopping exercises may be an even more unusual activity for inactive women, thus having an even greater osteogenic potential on the specific skeletal sites that come under strain.
A series of studies have recently been conducted to assess a programme of multidirectional hopping exercises in terms of GRF, feasibility and overall effectiveness with the aim of using such a high-impact unilateral exercise intervention to study the optimal exercise prescription for healthy premenopausal women. First, it was found that the GRF acting on one leg during a maximal vertical hop (mean 3·7 (sd 0·4)×body weight) exceeds the GRF acting on one leg during a maximal jump (mean 2·1 (sd 0·4)×body weight per leg, assuming symmetrical distribution of body weight on each foot)(Reference Bailey, Parsons and Brooke-Wavell62). Despite a lower hop height (mean 132 (sd 18) mm for a hop v. mean 241 (sd 46) mm for a jump), the GRF values recorded were found to be greater than those measured previously in a study that reported an increase in BMD at the trochanter following a jumping programme(Reference Bassey, Rothwell, Littlewood and Pye47). These comparisons indicate that hopping generates sufficient GRF to potentially provoke gains in BMD. Cross-sectional analyses of subsequent baseline data support this conclusion by demonstrating positive associations between GRF and bone indices, in most cases more than muscular strength (Fig. 4).
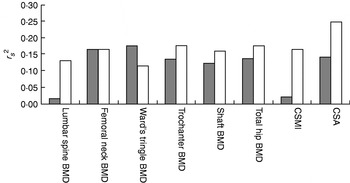
Fig. 4. Proportion of variance (r s2) in bone variables explained by isometric knee extensor strength (![]() ) and ground-reaction forces (□) in sedentary premenopausal women. BMD, bone mineral density; CSMI, cross-sectional moment of inertia; CSA, cross-sectional area. (From Bailey et al.(Reference Bailey, Parsons and Brooke-Wavell62))
) and ground-reaction forces (□) in sedentary premenopausal women. BMD, bone mineral density; CSMI, cross-sectional moment of inertia; CSA, cross-sectional area. (From Bailey et al.(Reference Bailey, Parsons and Brooke-Wavell62))
Second, a pilot study has indicated that performing an intervention including a warm-up and hopping exercises in the long term would be feasible, as evidenced by 99% compliance with a 6-week-long intervention (CA Bailey and K Brooke-Wavell, unpublished results). Importantly, no injuries were sustained and only one of the twelve participants experienced muscle soreness, which was short lived. The high compliance rate may be a result of the low demands of the programme, because unlike many exercise studies the participants were not required to spend time travelling to a specific location to perform the training in a group under supervision(Reference Friedlander, Genant, Sadowsky, Byl and Gluer63, Reference Heinonen, Oja, Sievanen, Pasanen and Vuori64). Instead they were free to decide where and when to do their hopping exercises, thereby making it a much easier task to fit into their daily routine.
Last, in terms of the effectiveness of hopping to increase BMD, the results of a 6-month-long intervention study, during which twenty-one sedentary premenopausal women performed an intervention that included fifty multidirectional hops between two and seven times per week, show that BMD at the femoral neck and lower neck of the hoppers' trained leg is increased by 1·04% (P=0·041) and 0·9% (P=0·048) respectively in comparison with their control leg(Reference Bailey, Parsons and Brooke-Wavell65) (Fig. 5). The ten controls, who continued their normal lifestyle, were not found to show changes in BMD at either site. As well as positive changes in BMD, changes were also found in hop height, muscle strength and balance, all factors that contribute to reduced falls and subsequent fractures. Thus, brief hopping exercises could have positive local effects on sites that are important in osteoporosis prevention.

Fig. 5. Changes in bone mineral density (BMD) following a 6-month high-impact unilateral intervention study during which twenty-one sedentary premenopausal women performed an intervention that included fifty multidirectional hops between two and seven times per week (A) and ten controls continued their normal lifestyle (B).![]() , Lumbar spine; (
, Lumbar spine; (![]() ), Trained leg; (□), control leg. Values are means with their standard errors represented by vertical bars. The difference between legs and between groups was significant: *P<0·05. (From Bailey et al.(Reference Bailey, Parsons and Brooke-Wavell65))
), Trained leg; (□), control leg. Values are means with their standard errors represented by vertical bars. The difference between legs and between groups was significant: *P<0·05. (From Bailey et al.(Reference Bailey, Parsons and Brooke-Wavell65))
Conclusions and implications
Physical activity has been shown to reduce skeletal fragility and predisposition to falls through a combination of increased BMD and improved physical capabilities such as coordination, balance, reaction time and muscle function. In the human model the determination of a dose–response relationship is hindered by a lack of the appropriate technology to directly evaluate mechanical loading and skeletal competence. However, results from both animal and human trials indicate that if strains are high in magnitude, rapidly-applied, dynamic and novel a considerable bone response can be achieved at the skeletal site coming under strain with remarkably short durations of loading. Prescribing a specific and efficient exercise programme for optimum bone health could therefore be based on these principles so that each individual maximises benefits from their time and effort spent on exercise. The issue of feasibility and practicality is paramount not only for adherence to research interventions but also to lifestyle intervention. The optimal exercise prescription still needs further definition. Moreover, more research among non-athletic premenopausal women, who are a more accurate reflection of the general population, is needed. High-impact unilateral exercise interventions may provide a useful method of assessing the relationship between mechanical loading and bone to more precisely define the most advantageous exercise prescription for optimising peak bone mass.








