Brain development is subject to complex lifelong processes of interactions between genetic and environmental factors. Although many environmental factors contribute to brain development, nutrition is of particular importance through the role that nutrients play in specific metabolic pathways and structural components( Reference Georgieff 1 ). It is known, for example, that a dietary deficiency during critical periods of development can result in permanent changes to the brain( Reference Anjos, Altmäe and Emmett 2 ). Of particular importance are folate and the related B-vitamins that are involved in C1 metabolism and in the production of S-adenosylmethionine (SAM), a universal methyl donor required for various reactions, including the production of neurotransmitters( Reference Dominguez-Salas, Cox and Prentice 3 ). B-vitamins are required for essential brain metabolic pathways and are fundamental in all aspects of brain development and maintenance of brain health throughout the lifecycle( Reference van de Rest, van Hooijdonk and Doets 4 ).
This review will explore the evidence linking maternal folate status with neurocognitive development in the offspring and will consider the associated explanatory mechanisms. In addition, the roles of B-vitamins and relevant genetic interactions in relation to cognitive health in older adults will also be examined.
The role of folate and related B-vitamins in C1 metabolism
Folate, a substrate of various enzyme reactions, along with vitamins B12, B6 and riboflavin in their co-factor forms, are involved in C1 metabolism, which comprises a network of interrelated biochemical pathways that donate and regenerate C1 units, including methyl groups (Fig. 1). Within the folate cycle tetrahydrofolate acquires a carbon unit from serine in a vitamin B6-dependent reaction, which subsequently forms 5, 10-methylenetetrahydrofolate. The latter is involved in the synthesis of thymidine, which in turn is incorporated into DNA or it is converted to 5-methyltetrahydrofolate which participates in a riboflavin (i.e. FAD)-dependent reaction, catalysed by the enzyme methylenetetrahydrofolate reductase (MTHFR). 5-Methyltetrahydrofolate then serves as single carbon donor, feeding into the C1 pathway by donating its methyl group to homocysteine to form methionine, via the vitamin B12-dependent enzyme methionine synthase. Methionine is a precursor for the synthesis of SAM, a methyl donor required for the methylation of DNA, proteins, chromosomes and phospholipids, production of myelin and the synthesis and activation of neurotransmitters, including catecholamine and serotonin( Reference Schaevitz, Berger-Sweeney and Ricceri 5 ). Upon donating its methyl group, SAM is converted to S-adenosylhomocysteine and homocysteine, which can be further metabolised in the transsulphuration pathway to form cysteine, a B6-dependent process, or remethylated back to methionine.
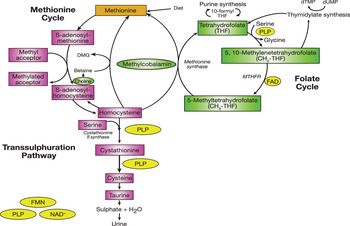
Fig. 1. (colour online) C1 metabolism. PLP, pyridoxal 5 phosphate; MTHFR, methylenetetrahydrofolate reductase; DMG, dimethylglycine; dTMP, deoxythymidine monophosphate; dUMP, deoxyuridine monophosphate. (Adapted from Clarke et al.( Reference Clarke, Ward and Strain 85 ))
It is important to note that some genetic factors may affect the function of enzyme activity, leading to disturbances in B-vitamin absorption, transport and uptake. Of particular interest is the common 677C→T polymorphism in the gene coding for MTHFR, and this polymorphism is an important genetic determinant of plasma homocysteine. Individuals with the homozygous mutant MTHFR 677TT genotype have reduced MTHFR enzyme activity. Homozygosity for this polymorphism alters B-vitamin requirements and has been linked to a number of degenerative diseases, including cognitive dysfunction( Reference Ford, Flicker and Hankey 6 ).
C1 metabolism, B-vitamins and early brain health
The role of folate in fetal brain development
Optimal folate status is essential throughout pregnancy for placental and fetal growth and development. During pregnancy there is a decline in maternal folate concentrations to approximately 50 % of non-pregnant concentrations( Reference McNulty, Pentieva and Marshall 7 ). This is partly owing to the increased folate requirements for rapid cell proliferation and tissue growth of the uterus and placenta, growth of the fetus and for the expansion of maternal blood volume( Reference McNulty, McPartlin and Weir 8 ). Irrefutable evidence has shown that supplementation with folic acid protects against both first occurrence( Reference Czeizel and Dudás 9 ) and recurrence( 10 ) of neural tube defects, leading to government recommendations, which are in place worldwide, advising all women planning a pregnancy to consume 400 μg folic acid/d from preconception until the end of the first trimester of pregnancy( 11 , 12 ). This protective effect of folic acid supplementation relates to the early stages of pregnancy when the closure of the neural tube occurs (about 21–28 d post-conception); however, little is known as to whether continuing folic acid usage throughout pregnancy confers any long-term beneficial effects to the offspring.
There is a growing body of evidence from observational and experimental studies, suggesting that nutrition in utero may affect later cognitive development in the offspring. As the fetal brain develops rapidly, poor maternal intake of key nutrients during pregnancy can influence the development of the structure and components of the brain. Folate and the metabolically related B-vitamins are fundamental throughout brain development via their participation in transcription, nucleotide synthesis, neurotransmitter production and methylation processes, including DNA methylation( Reference Reynolds 13 ).
Evidence linking maternal folate status with offspring cognitive performance
In recent years, the association between maternal folate and/or related B-vitamin status with later cognitive performance of the offspring has become a topic of interest. To date most of the literature in this area is derived from observational (Table 1) and animal studies. Given the recognised protection of folic acid in the prevention of neural tube defect during early fetal development, the majority of these studies reported peri-conceptional use of folic acid by women and are focused in the early stages pregnancy. Only a few studies have investigated maternal folate status in the second and third trimesters of pregnancy, i.e. after the recommended period, in relation to later cognitive performance of the child, and no available human evidence has addressed this question in a randomised trial in pregnancy.
Table 1. Observational studies investigating the association between maternal folate status with cognitive performance of the offspring

FA, folic acid; GW, gestational week; PPVT, Peabody Picture Vocabulary Test; WRAVMA, Wide Range Assessment of Visual Motor Abilities; DDST, Denver Development Screening Test; MSCA, McCarthy Scales of Children's Abilities; KABCTM -II, Kaufman Assessment Battery, 2nd edition; Bayley-III, Bayley Scales of Infant and Toddler Development, 3rd edition.
A number of human studies have shown positive associations between self-reported folic acid supplement use during the first trimester of pregnancy and cognitive performance of the child( Reference Julvez, Fortuny and Mendez 15 , Reference Roth, Magnus and Schjølberg 17 – Reference Villamor, Rifas-Shiman and Gillman 19 ). Julvez et al. ( Reference Julvez, Fortuny and Mendez 15 ) demonstrated that folic acid use in pregnancy was associated with improved neurodevelopment, verbal performance and motor development in children aged 4 years. Similarly, a large longitudinal study (n 1210) examined the association between maternal intake of methyl donor nutrients, including folate and vitamin B12, during the first trimester of pregnancy in relation to the cognitive performance of the offspring. The authors estimated that each 600 μg/d increment in total folate intake (from food and supplements combined) was associated with a 1·6 point higher score in cognitive performance in the child, assessed at age 3 years( Reference Villamor, Rifas-Shiman and Gillman 19 ). Likewise, another observational study indicated significantly higher verbal comprehension, vocabulary development and communication skills in Greek children born to mothers taking high dose folic acid (5 mg/d) in pregnancy, compared with those whose mothers did not take supplements( Reference Chatzi, Papadopoulou and Koutra 18 ). Most notably, evidence from a large prospective study (n 38 954) in the US-linked maternal folic acid supplement use from 4 to 8 weeks after conception with a reduced risk of severe language delay evaluated by self-reported parental questionnaires assessing the grammar of the child, at age 3 years( Reference Roth, Magnus and Schjølberg 17 ).
During the growth spurt in pregnancy (about 24–42 gestational weeks), the developing brain, owing to the sequence of developmental stages, including neuronal proliferation and myelination, is particularly vulnerable to adequate nutrition( Reference Isaacs 20 ). Throughout the late fetal and early postnatal periods areas such as the hippocampus, auditory and visual cortices and the striatum undergo rapid growth by morphogenesis and synaptogenesis which makes them functionally active( Reference Thompson and Nelson 21 ). All nutrients are important for brain development, but it is proposed that certain nutrients, including folate, have greater effects during the late fetal development. Only a few studies, however, have investigated the impact of maternal folate status in the later stages of pregnancy on the child's neurodevelopment. Nearly 40 years ago, Gross et al. ( Reference Gross, Newberne and Reid 14 ) reported that children born to mothers with diagnosed megaloblastic anaemia during the third trimester of pregnancy had abnormal neurodevelopment and lower intellectual abilities compared with infants born to mothers with optimal folate status. Several decades later a study investigating the impact of maternal blood folate, B12 and homocysteine concentrations, at 30 gestational weeks, in relation to cognitive performance of 9–10-year-old children (n 536), showed that higher maternal folate status during late pregnancy predicted better cognitive performance in children( Reference Veena, Krishnaveni and Srinivasan 16 ). No association was observed for maternal B12 or homocysteine status on the overall cognitive ability of the children( Reference Veena, Krishnaveni and Srinivasan 16 ).
To date no randomised controlled trial (RCT) has investigated the effect of maternal folate status during the later stages of pregnancy and subsequent cognitive performance of the offspring. A pilot study (n 39), conducted by our group, examined the effect of folic acid supplementation in the second and third trimester of pregnancy and subsequent cognitive performance of the child. The novel results showed that children (aged 3 years) born to mothers supplemented with folic acid, scored significantly higher in the cognitive domain of the Bayley's development assessment( Reference Pentieva, McGarel and McNulty 22 ). These highly promising results from this RCT now need to be confirmed on a larger scale.
Mechanistic studies in animal models also provide evidence that prenatal folate deficiency may be causally related to adverse structural changes in the brain( Reference Whitley, O'Dell and Hogan 23 , Reference Craciunescu, Brown and Mar 24 ). Craciunescu et al. ( Reference Craciunescu, Brown and Mar 24 ) reported that the offspring of rats fed a folic acid deficient diet during days 11 and 17 of gestation (i.e. corresponding to mid- and late stages of human pregnancy), had a reduction in progenitor cells in the fetal neocortex (an area responsible for complex behaviours, including cognition) suggesting that the developing fetal brain is vulnerable to maternal folate deficiency, which may adversely affect cognitive performance in the later life( Reference Craciunescu, Brown and Mar 24 ). Similarly, another study showed that gestational B-vitamin deficiency resulted in an accumulation of homocysteine with ‘concomitant apoptosis’ in selective brain areas, the cerebellum, striatum and hippocampus, which altered motor function and learning and memory abilities in rats( Reference Blaise, Nédélec and Schroeder 25 ).
Evidence regarding the impact of maternal folate status on the offspring neurodevelopment is further strengthened by genetic studies. The common 677C→T polymorphism in the gene coding for the folate metabolising enzyme MTHFR is an important genetic determinant of plasma homocysteine and individuals homozygous for the polymorphism (MTHFR 677TT genotype), are prone to low folate status and elevated plasma homocysteine concentrations. Recent studies from Mexico, where the frequency of the MTHFR 677TT genotype is reported to be the highest in the world( Reference Wilcken, Bamforth and Li 26 ), showed that the maternal MTHFR 677TT genotype is a predictor of poor child neurodevelopment( Reference Pilsner, Hu and Wright 27 ) especially in combination with low maternal folate intake (<400 μg/d) during the first trimester of pregnancy( Reference del Río Garcia, Torres- Sanchez and Chen 28 ). However, research in this area is limited and further studies are required to investigate whether children born to mothers genetically susceptible to impaired folate status are more at risk of impaired neurodevelopment.
Not all studies support the association between maternal folate status and the neurodevelopment of the child. A Hungarian RCT investigating pregnant women consuming a multi-vitamin containing folic acid (0·8 mg/d) before conception until the second month of pregnancy did not find any evidence of improved ‘mental development’ of children aged 6 years( Reference Dobó and Czeizel 29 ). The present study, however, was primarily designed to investigate the effect of folic acid on neural tube defects during the very early stages of pregnancy. Likewise a longitudinal study found no evidence of an association between maternal blood folate status (low ≤11 nmol v. normal >11 nmol/l) in the later stages of pregnancy (19, 26 and 37 gestational weeks) and cognitive performance of children aged 5 years( Reference Tamura, Goldenberg and Chapman 30 ). Possible reasons to explain these inconsistencies may include the influence of socio-economic status, a well-known confounder for cognitive development and which could potentially confound any effect of folic acid( Reference Tamura, Goldenberg and Chapman 30 ). Furthermore, the studies by Dobo & Czeiel( Reference Dobó and Czeizel 29 ) and Tamura et al. ( Reference Tamura, Goldenberg and Chapman 30 ) used a multi-vitamin approach, rather than folic acid alone; an approach, which again could impact the findings.
Overall, the observational and animal evidence appears to be supportive for a role of maternal folate status in later cognitive performance of the child. Aside from cognitive health, there are also studies linking low maternal folate status with higher offspring behavioural( Reference Roza, van Batenburg-Eddes and Steegers 31 ), inattention and hyperactivity problems( Reference Schlotz, Jones and Phillips 32 ) and emotional problems( Reference Steenweg-de Graaff, Roza and Steegers 33 ), which warrant further investigation. It is important to note however that much of the observational evidence is based on self-reported folic acid usage, usually during the early fetal development stages( Reference Julvez, Fortuny and Mendez 15 , Reference Roth, Magnus and Schjølberg 17 – Reference Villamor, Rifas-Shiman and Gillman 19 ), with only a few studies exploring the effect of supplementation during later stages of pregnancy( Reference Gross, Newberne and Reid 14 , Reference Veena, Krishnaveni and Srinivasan 16 ) and none doing so using a RCT. Considering that there is rapid structural and synaptic development in key areas of the brain, including the hippocampus, during the growth spurt in pregnancy, further studies are required to more fully investigate the potential effect of folate throughout pregnancy and to determine if the effect is specific to certain stages of pregnancy or perhaps the effect is mediated throughout all trimesters of pregnancy.
Other evidence supporting folate status in relation to brain health in the young
The effect of dietary and blood folate status on cognitive performance has also been investigated in young children and adolescents. A recent study by Strand et al. ( Reference Strand, Taneja and Ueland 34 ) reported that low plasma folate and vitamin B12 concentrations were associated with poorer cognitive performance, measured in children aged 12–18 months. Furthermore, an investigation of Swedish adolescents (age 15 years) showed that higher dietary folate intakes was positively associated with academic achievements( Reference Nilsson, Yngve and Bottiger 35 ). Nguyen et al. ( Reference Nguyen, Gracely and Lee 36 ) also reported that higher serum folate measured in children aged 6–16 years was associated with higher performance in reading and also in block design in participants from the National Health and Nutrition Examination Survey III cohort in the USA.
C1 metabolism, B-vitamins and brain health in ageing
Cognitive dysfunction and dementia
Cognitive dysfunction is a common problem among the ageing population and ranges in severity from mild cognitive impairment to more progressive types of dementia; the latter referring to a state in which the disease is sufficient to impair normal way of living( Reference Graham, Rockwood and Beattie 37 , Reference McNulty and Scott 38 ). Although some degree of cognitive decline is considered a normal and an unpreventable aspect of ageing, the development of dementia and Alzheimer's disease is attributable to diseases of the brain, resulting in changes to the brain structure sufficient to interfere with normal life activities. Globally an estimated 35·6 million people suffer from dementia, affecting 7 % of individuals aged over 65 years and 30 % of those over 80 years( 39 ). Given the increase in life expectancy, these figures are expected to double worldwide by 2025( 39 ) and represents a major public health challenge for future generations.
As brain changes start to progress long before the diagnosis of dementia is overt, it is important to find early biomarkers that would enable timely interventions to delay the onset or slow the progression of the disease( Reference Kivipelto, Ngandu and Laatikainen 40 ). Well-established non-modifiable risk factors include increasing age, family history and genetic factors. However, evidence has now amassed from long-term epidemiological studies linking potentially modifiable lifestyle factors, including smoking status, physical inactivity and nutritional factors with cognitive dysfunction. Emerging evidence suggests that suboptimal status of folate and the metabolically related B-vitamins and/or elevated homocysteine concentrations, owing to their essential roles in C1 metabolism may be linked with cognitive dysfunction and dementia.
Evidence linking B-vitamins with brain health in ageing
The majority of epidemiological studies generally support an association between suboptimal status of folate, the metabolically related B-vitamins or elevated concentrations of the metabolite homocysteine with cognitive dysfunction in older adults. Indeed, a review by Smith ( Reference Smith 41 ) some years ago reported that 90 out of 100 published cross-sectional and prospective studies showed a link between elevated homocysteine and/or low B-vitamins concentrations with cognitive dysfunction. Most of these studies have focused on elevated plasma homocysteine concentrations( Reference Ravaglia, Forti and Maioli 42 – Reference Miller, Green and Ramos 46 ), and/or a combination of suboptimal status of folate and vitamin B12 ( Reference Ramos, Allen and Mungas 47 – Reference Hooshmand, Solomon and Kåreholt 54 ) and to a much lesser extent on vitamin B6 ( Reference Riggs, Spiro and Tucker 55 , Reference Moorthy, Peter and Scott 56 ). To the authors’ knowledge, no published study thus far has focused on riboflavin alone as a potential contributor to cognitive health. Notably, there are a number of limitations associated with these studies; typically, only one cognitive assessment tool was used, rather than a battery of tests providing information for multiple cognitive domains; depression and anxiety (known confounders) are not measured and there is a lack of data on vitamin supplement usage, all of which can limit the reliability of reported results.
It is also important to take into consideration recent concerns regarding mandatory folic acid fortification and the potential ‘masking’ effect of vitamin B12 deficiency among older adults. In B12 deficiency, the activity of methionine synthase is reduced; therefore 5, methyltetrahydrofolate cannot be converted to tetrahydrofolate (the active form of folate) and becomes trapped in an unusable form. Evidence from the National Health and Nutrition Examination Survey reported that although higher folate was generally associated with better cognitive health, a combination of high plasma folate (>59 nmol/l) with low plasma B12 (<148 pmol) and elevated methylmalonic acid (a B12-specific functional biomarker), was actually associated with poorer cognitive performance compared with individuals with normal concentrations of these biomarkers( Reference Morris, Jacques and Rosenberg 57 ). Furthermore, Moore et al. ( Reference Moore, Ames and Mander 58 ) showed participants with high red cell folate and low serum B12 were three times more likely to have impaired cognitive performance. In contrast, however, analysis from the Hordaland Health study failed to confirm this association in their analysis( Reference Doets, Ueland and Tell 59 ). The disagreement between studies is possibly linked to the relatively small sample of participants with the combination of high folate and low B12 status available for analysis in these studies. In addition, high and low status of folate and B12 is defined differently among various published studies.
Following the positive associations from epidemiological evidence a number of RCT have investigated the potential benefits of B-vitamin supplementation on cognitive health (Table 2). Many of these trials however were of insufficient power and duration to detect an effect or included participants with existing optimal B-vitamin status or with advanced dementia, where a beneficial effect is not likely( Reference McMahon, Green and Skeaff 60 , Reference Aisen, Schneider and Sano 62 , Reference Lewerin, Matousek and Steen 67 – Reference Kwok, Lee and Law 70 ).
Table 2. Summary of randomised trials assessing the effect of B-vitamin treatment on cognitive function in older adults

FA, folic acid; hcy, homocysteine; AD, Alzheimer's disease; MCI, mild cognitive impairment.
Notably, two similarly designed homocysteine-lowering trials have yielded conflicting results. The Folic Acid and Carotid Intimamedia Thickness (FACIT) trial found that supplementation with 800 μg folic acid/d over 3 years in healthy adults’ (n 818) improved global cognitive function, in particular memory, information processing speed and sensorimotor speed( Reference Durga, van Boxtel and Schouten 61 ). In contrast, a 2-year trial also conducted in healthy individuals (n 276) supplemented with a combination of folate (1000 μg/d), B12 (500 μg/d) and B6 (10 mg/d) or placebo, reported no significant effect on cognitive performance, across the eight cognitive assessments used( Reference McMahon, Green and Skeaff 60 ). Although these studies were of similar design, sufficiently powered, had comparable baseline cognitive scores and used similar exclusion criteria, it is important to note the difference in the baseline folate status of participants between these two trials. The baseline folate (12 nmol/l) was lower in the FACIT trial compared with the McMahon study (baseline folate 22·6 nmol/l), which may suggest that any benefits of folic acid on cognitive health in ageing may arise from correcting suboptimal folate status, whereas supplementing with additional folic acid to those with optimal status may not provide any further benefit to cognitive function. More recently, a significant improvement in overall cognitive performance was found following a 2-year intervention with B-vitamins in participants with elevated psychological distress( Reference Walker, Batterham and Mackinnon 64 ). The majority of the reported intervention trials used questionnaire-based assessments of cognitive performance; very few clinical trials have investigated the effect of B-vitamins on cognitive dysfunction using direct methods, such as brain imaging techniques, which may provide a more robust measure of long-term brain changes, including the impact of nutritional interventions.
The strongest evidence to date originates from the VITACOG trial in Oxford, in which patients with mild cognitive impairment (without dementia) were supplemented with folic acid (800 μg/d), B12 (500 μg/d) and B6 (20 mg/d) or placebo over a 2-year period. The results reported that supplementation with B-vitamins slowed the rate of cognitive decline, assessed by questionnaire-based cognitive tests( Reference de Jager, Oulhaj and Jacoby 63 ). Of greater importance is the fact that the same study also found that B-vitamin treatment reduced brain atrophy, as measured by MRI scans, by approximately 30 %( Reference Smith, Smith and de Jager 65 ). The effect was greatest in participants with the highest baseline homocysteine concentrations (>13 μmol/l), among whom an overall reduction of brain atrophy rate of 53 % was reported, while no effect was found in those in the bottom quartile (≤9·5 μmol/l)( Reference Smith, Smith and de Jager 65 ). Moreover, when a subsample of VITACOG cohort were further analysed, the investigators reported that B-vitamin treatment reduced the cerebral atrophy, by as much as 7-fold, specifically in grey matter areas of the brain vulnerable to Alzheimer's disease, including bilateral hippocampus and cerebellum( Reference Douaud, Refsum and de Jager 66 ). Finally, when participants were analysed by quartiles for brain shrinkage, it was reported that participants with the highest rate of brain shrinkage displayed the most cognitive decline( Reference de Jager 71 ). Importantly, folate, vitamin B12 and vitamin B6 are all required to lower concentrations of homocysteine and perhaps it is the combination of B-vitamins, and not a monotherapy B-vitamin approach, which is required to optimise C1 metabolism. This research has paved the way for future RCT in this area; more research is however warranted in both healthy and cognitively impaired groups to investigate the proposed effect further.
In summary, results from large observational studies conducted in healthy and cognitively impaired cohorts provide strong evidence of a possible relationship between suboptimal B-vitamin status, elevated homocysteine (independently or as a marker of B-vitamin status) and cognitive dysfunction. At present the most promising evidence supporting a cause and effect relationship comes from the VITACOG trial( Reference de Jager, Oulhaj and Jacoby 63 , Reference Smith, Smith and de Jager 65 , Reference Douaud, Refsum and de Jager 66 , Reference de Jager 71 ). These findings now require replication in other population groups to confirm the role played by B-vitamins in cognitive health. If cognitive dysfunction can in fact be slowed or prevented by B-vitamins, in healthy older people, then this could offer a cost-effective preventative public health strategy in ageing populations.
Potential mechanisms linking B-vitamins with brain health
Potential mechanism explaining the role of B-vitamins in early brain development
B-vitamins appear to have direct roles on brain development and maintenance, through their involvement in C1 metabolism. A new emerging area of research concerns the role of epigenetics, which is broadly defined as the ‘heritable changes in gene function that cannot be explained by changes in the DNA sequence’( Reference Russo, Martienssen and Riggs 72 ). Epigenetic alternations in utero may have the potential to programme diseases in adulthood. Studies have recently begun to investigate whether epigenetic modification, through nutritional interventions, can influence an individual's health in later life. DNA methylation is the most widely studied epigenetic mechanism and occurs through C1 metabolism, which is dependent on folate and several cofactors, including the related B-vitamins.
A number of animal studies have addressed the issue of maternal folate status and subsequent epigenetic effect on the offspring. Waterland & Jirlte( Reference Waterland and Jirtle 73 ) demonstrated that in pregnant agouti mice a high methyl diet, resulted in offspring with mottled brown coats, which were less obesogenic and less prone to diseases. Interestingly, methyl donor supplementation of agouti pregnant dams appeared to not only affect the fetus, but also the subsequent generation, suggesting that the maternal diet may influence several generations( Reference Cropley, Suter and Beckman 74 ). Recently, Cho et al. ( Reference Cho, Sánchez-Hernández and Reza-López 75 ) also provided evidence of the epigenetic effects of folate supplementation in pregnancy and showed that a high folate diet throughout pregnancy and weaning resulted in dams less exposed to an obseogenic phenotype. Collectively, these studies show that restriction in folate can influence DNA methylation in the offspring and in turn influence gene expression and disease related phenotypes.
The results from human studies, although limited, are also generally supportive of the effect of folate on epigenetic processes. Evidence from the Dutch Hunger Winter study has shown that malnutrition (in this case because of extreme famine) during pregnancy may induce permanent epigenetic changes in IGF2 ( Reference Roseboom, Painter and van Abeelen 76 , Reference Heijmans, Tobi and Stein 77 ). Recent studies suggest that IGF2 epigenetic changes in utero are associated with long-term metabolic health risk in human subjects( Reference Heijmans, Tobi and Stein 77 ). The results from Steegers-Theunissen et al.( Reference Steegers-Theunissen, Obermann-Borst and Kremer 78 ) showed that reported maternal folic acid supplement usage was associated with an increased methylation (4·5 %) of IGF2 differentially methylated region of the offspring, 17 months after delivery. Moreover, maternal SAM concentrations were related to the offspring IGF2 differentially methylated region's methylation levels, indicating that the maternal environment had a greater influence on the methylation of IGF2 in the offspring( Reference Steegers-Theunissen, Obermann-Borst and Kremer 78 ). In further support, results from an observational study (n 913) investigating the effect of folic acid use after the recommended 12 gestational weeks, reported that supplement use was associated with significant, albeit small, elevation in IGF2 methylation and reduced methylation in paternally expressed 3 (PEG3) and LINE-1( Reference Haggarty, Hoad and Campbell 79 ). No effect was observed before 12 weeks gestation; perhaps suggesting an epigenetic effect of folate occurred during the later stages of pregnancy. Considering that IGF2 is involved in placental and fetal development and PEG3 is a gene highly expressed in the brain and also involved in the development of the fetal hypothalamus( Reference Ivanoca and Kelsey 80 , Reference Keverne 81 ), alterations in the methylation of these genes may subsequently exert influence on their expression, which in turn might have an impact on the offspring's brain development. However, further studies are required to expand on the current knowledge of nutrition and disease prevention through epigenetic mechanisms.
Potential mechanism explaining the role of B-vitamins with brain health in ageing
Mechanisms linking B-vitamins and cognitive health in ageing are speculative and several hypotheses have been suggested. Folate and vitamin B12 are required for the production of SAM, which in turn is required for various methylation reactions and adequate production of neurotransmitters. Deficiencies in these vitamins may lead to disturbances in important methylation reactions, which may affect pathways associated with cognitive health. It is also proposed that deficiencies in folate, vitamins B12 and B6 can disrupt the remethylation of homocysteine to methionine, resulting in hyperhomocysteinemia. Studies have linked elevated plasma homocysteine concentrations with atrophy of the hippocampus; an area of the brain required for memory consolidation( Reference den Heijer, Vermeer and Clarke 82 ). It is therefore suggested that high homocysteine concentrations may have direct neurotoxic effects, resulting in apoptosis and possibly impairing pathways associated with cognition( Reference Walker, Batterham and Mackinnon 64 , Reference Clarke, Smith and Jobst 83 ). In addition, optimal B6 status plays an important role in brain health through its essential role in transamination and decarboxylation reactions required for the metabolism of several neurotransmitters, including serotonin, γ-aminobutyric acid, dopamine, noradrenaline and histamine( Reference di Salvo, Contestabile and Safo 84 ).
Conclusion
Given the importance of C1 metabolism in a wide array of processes, including the production of neurotransmitters and DNA methylation, it is not surprising that disturbances in this cycle may have profound effects on both the developing and ageing brain. Mechanistically it is clear that folate and the related B-vitamins are critical for brain function throughout the lifecycle. Some important questions, however, still need to be considered. The potential role between maternal folate status during pregnancy and later neurodevelopment of the offspring needs to be explored through well-designed RCT. A clearer understanding of the role of folate in early brain health will help to inform future policies in relation to folic acid recommendations in pregnancy. Compelling evidence from epidemiological studies and RCT show that there is a strong association between suboptimal B-vitamin status and/or elevated homocysteine with an increased risk of cognitive dysfunction in older adults. Further research from well-conducted RCT which includes brain-imaging techniques is warranted to shed light on some fundamental questions. In conclusion, current evidence suggests that folate and the metabolically related B-vitamins may be important contributors to brain health across the lifecycle. Improving knowledge of potential epigenetic mechanisms during pregnancy and postnatal life will help provide the important mechanistic links between B-vitamins and brain health.
Financial Support
This work was supported by the funding from the Northern Ireland Department for Employment and Learning which funded the PhD studentship for C. M. G. The Northern Ireland Department for Employment and Learning had no role in the design, analysis or writing of this article.
Conflict of Interest
None.
Authorship
C. M. G. drafted the manuscript. K. P., H. McN. and J. J. S. critically revised the manuscript for important intellectual content. All authors have read and approved the final manuscript.





