The number of people living beyond a cancer diagnosis in the UK is currently estimated at 2 million people(Reference Maddams, Utley and Møller1). It is predicted to increase in the UK and estimated that by 2024 nearly a quarter of people aged over 65 years will be a cancer survivor(Reference Maddams, Utley and Møller1). These figures are mirrored internationally with the number of people surviving cancer currently at 14·5 million in North America, which is set to reach 19 million by 2024(Reference DeSantis, Lin and Mariotto2). People are living longer after a cancer diagnosis due to a higher incidence of cancers being diagnosed and treated in the ageing population combined with improvements in anticancer therapies(Reference DeSantis, Lin and Mariotto2). The health and wellbeing of cancer survivors has subsequently become an important topic for both healthcare professionals and researchers(Reference Virgo, Bromberek and Glaser3). Healthcare needs of those who have survived cancer reflect the needs of those in other chronic diseases and are often complex(Reference Elliott, Fallows and Staetsky4, Reference Leach, Weaver and Aziz5). It is therefore becoming essential to act to meet the needs of this growing population due to the estimated increased demand for healthcare resources and societal impact(Reference Fenn, Evans and McCorkle6, Reference Lorgelly and Neri7). These include direct health care costs, out of pocket costs for patients and their families, informal carer costs and productivity losses as well as decreased health related quality of life(Reference Lorgelly and Neri7).
Even though there are similarities with other chronic conditions, people living after cancer have been shown to have increased levels of motivation for a healthier life style(Reference Spector8). Experiencing cancer is often seen as a critical life changing event that acts as a catalyst for people to change their priorities(Reference Stanton, Rowland and Ganz9) and 50–80 % of post-treatment cancer survivors have been shown to make positive lifestyle modifications(Reference Stanton, Bower, Low, Calhoun and Tedeschi10). This is often termed the ‘teachable moment’ and refers to a time healthcare professionals may be able to positively intervene coined ‘riding the crest of the wave’(Reference Demark-Wahnefried, Aziz and Rowland11). This has led to a surge of research targeting this time point aiming to capitalise on increased motivation required for altering lifestyle behaviours in relation to both diet and activity(Reference Lee, Ozakinci and Leung12–Reference Bluethmann, Basen-Engquist and Vernon14).
Increased motivation or intention to act is not always translated into healthier lifestyle choices, as has been demonstrated in a large prospective study showing that people after cancer did not change their behaviour in relation to smoking, alcohol or physical activity(Reference Williams, Steptoe and Wardle15). This indicates that more needs to be done to capitalise on motivation with specific targeted interventions. Diet is part of a healthy lifestyle and there are specific nutritional recommendations for the prevention of cancer that are mirrored for people living after cancer(16). Risk of death is inversely related to these recommendations and this has been demonstrated in an analysis of 378 863 participants that shows people who followed a greater number of these recommendations had a 34 % lower hazard ratio then those following only a few(Reference Vergnaud, Romaguera and Peeters17).
The association between dietary intake and a number of cancers is now well established from epidemiological data on dietary patterns associated with elevated risk(16).
High fat, low dietary fibre, low consumption of fruit and vegetables and high refined carbohydrate are well documented dietary patterns associated with increased risk(Reference Gonzalez and Riboli18–Reference Bradbury, Appleby and Key20). A high BMI has also been shown to increase risk of some cancers(Reference Lahmann, Hoffmann and Allen21). Recommendations are based on large cohort studies and meta-analyses for the prevention of cancer and these recommendations are also relevant for people who have survived a cancer diagnosis. However, evidence from randomised control trials (RCT) demonstrating clinical benefit from dietary interventions in terms of mortality and morbidity are limited in cancer survivors(Reference Pierce, Natarajan and Caan22). Nonetheless, there have been some positive outcomes for cancer survivors with regard to lowering BMI and improvements have been shown in dietary intake and anthropometry, aligned to cancer prevention recommendations(Reference Robien, Demark-Wahnefried and Rock23).
The rationale for dietary manipulation alone or in conjunction with lifestyle modifications is based not only on evidence for cancer prevention but also on information relating to individuals’ experiences of living beyond cancer. People who have survived cancer have a higher incidence of CVD, diabetes and secondary malignancies after cancer and treatment(Reference Hewitt, Rowland and Yancik24, Reference Khan, Watson and Rose25), and 47 % were found to have at least one or more co-morbidities in addition to cancer(Reference Elliott, Fallows and Staetsky4, Reference Søgaard, Thomsen and Bossen26). The occurrence of at least one or more chronic conditions was higher in those after cancer compared with age–sex matched controls with the greatest differences in heart disease, respiratory disorders, psychological disorders and urogenital conditions(Reference Jabaaij, van den Akker and Schellevis27). CVD is greater in people after anti-cancer therapy and this is now well established in a number of studies, which concentrate on different cancer sites including breasts, endometrial, testicular, brain and head and neck tumours(Reference Mulrooney, Blaes and Duprez28–Reference El-Fattah30). On-going heath care problems that exist secondary to cancer and its treatments can now be assessed using validated tools such as the Cancer Survivors Core Set to allow identification of health care issues(Reference Geerse, Wynia and Kruijer31).
A greater number of general practitioners visits, more medical prescriptions and home visits were observed in people who had a cancer diagnosis when compared with controls(Reference Jabaaij, van den Akker and Schellevis27). The combination of a higher rate of co-morbidities and an increased need for utilising healthcare resources leads to increase costs for the provision of healthcare for those who have survived cancer(Reference Khan, Watson and Rose25).
The rationale for promoting healthy lifestyles in people who have survived cancer has been outlined with justifications based on clinical outcomes from cohort studies and evidence gained from epidemiological studies on diet and cancer(Reference Velentzis, Keshtgar and Woodside32). Thus further discussion in this review will develop to give an overview of data surrounding motivation for change in cancer survivors and individuals’ preferences for the provision of dietary interventions after cancer. Finally, a narrative overview is presented of RCT on dietary interventions for people after a cancer diagnosis.
Motivation for change
It has been established that people living beyond cancer change their dietary intake and seek information to enable them to make healthier lifestyle choices. Women living after breast cancer were found to increase their intake of fruit and vegetables, whole grains and lean sources of protein, and decreased their intake of fat, sugar, red meat, coffee and some alcoholic drinks(Reference Velentzis, Keshtgar and Woodside32). The use of supplements also increased to 62 %, albeit, 56 % of women prior to diagnosis were already taking a supplement; those most frequently taken were fish oils, multivitamin and minerals, and evening primrose oil(Reference Velentzis, Keshtgar and Woodside32). Dietary modifications were observed in women after being diagnosed with breast cancer in Malaysia; two-thirds were found to have decreased energy, protein, total fat and vitamin E, and increased carotene and vitamin C(Reference Shaharudin, Sulaiman and Shahril33). Similar results have been found in people who survived cancer in the USA and UK(Reference Patterson, Neuhouser and Hedderson34, Reference Maskarinec, Murphy and Shumay35). Women with a diagnosis of breast cancer were motivated to change dietary behaviours because they were advised by their doctor, they received advice from a dietitian or stated they wanted to help cure their cancer(Reference Shaharudin, Sulaiman and Shahril33).
Qualitative evidence
From semi-structured interviews in people living after colorectal, breast and prostate cancer it has been found that people actively engage in meal planning and healthy food choices after surgery. Whilst, others engaged family members for support or focused on dietary health messages about decreasing fat and increasing fruit and vegetables(Reference Burden, Stamataki and Hill36, Reference Williams and Jeanetta37). Conversely, a minority reported that they were reluctant to engage in any dietary modification(Reference Burden, Stamataki and Hill36). From these interviews people after a diagnosis of colorectal cancer were classified as active, reluctant and resistant changers. Those classed as active changers were knowledgeable about healthy eating and some mentioned dietary messages specific to colorectal cancer quoting advice to decrease processed meat products and red meat, whilst others took a more holistic approach to their diet involving multiple food groups. Those classed as reluctant changers reported that they felt change was necessary. However, they embraced dietary modification with a degree of apprehension and out of a sense of need. Those classed as resistant changers did not report any diet or lifestyle changes and justified their reasoning.
The majority of those interviewed were positive about dietary modifications and were classified as active changers having made positive behavioural changes; only a few had not changed their behaviour, and some reluctant changers indicated they had made changes, albeit out of necessity or family pressure. Quotations that are reflective of responses for the active, reluctant and resistant changers are shown in Table 1. The quotations demonstrate that people after cancer were knowledgeable about dietary intake and cancer prevention and had actively engaged in positive change. The common food groups changed included red meat, processed foods, fruit and vegetables. Quotations from cancer survivors support other documented evidence of high levels of motivation in people after cancer relating to dietary behaviours(Reference Williams and Jeanetta37).
Table 1. Quotations from people after colorectal cancer

What are cancer survivors’ preferences for healthy eating interventions?
It is acknowledged that a large proportion of people seek information after a cancer diagnosis, although few studies have asked survivors their preferences for obtaining information. One method of eliciting peoples’ preferences for healthy eating and lifestyle advice is by using discrete choice experiments (DCE). Best worst choice DCE is a research method derived from health economics that determine which aspects of healthcare delivery are preferred by the user(Reference Clark, Determann and Petrou38–Reference Flynn40). They use a set of scenarios with difference levels, which can include how, where, who and arbitrary costs that denote willingness to pay.
People's preferences for dietary interventions after colorectal cancer (n 179) were explored with DCE. The responses showed that most participants’ preferred dietary advice provided by a bowel specialist nurse, at hospital and by an individual discussion either face-to-face or on the telephone(Reference Wright, Gibson and Eden41). The majority of participants did not like the scenario where advice was given by a general nurse, by email or in a group. From further analysis of the best worse DCE it was noted that there were inconsistencies within the data that were evident by contradictory findings between the best and worst preferences indicated by participants.
These contradictory findings were explored and showed that different groups could be identified within the cohort of colorectal cancer survivors. People who indicated they were meeting most of the dietary recommendations and indicated they were low risk, were most likely to be young males and indicated they preferred to receive dietary information via email in their own home. People who were older preferred to access services locally and preferred one-to-one advice and were strongly averse to receiving information via email. Whereas, people who reported they were following fewer healthy eating recommendations preferred direct in-person communication at their own doctor's surgery and were averse to email and telephone modes of provision(Reference Wright, Gibson and Eden41).
DCE are an innovative way of exploring people's preferences for healthcare and demonstrate that different people had a variety of preferences for service attributes. This is important information as aligning service provision to cancer survivors’ preferences may lead to increased uptake and effectiveness of interventions.
Dietary interventions
Dietary interventions that have been evaluated in the literature for people who have survived cancer include group sessions, face-to-face sessions, telephone and mailed interventions. Dietary interventions have focused on either weight management strategies or healthy eating recommendations.
This narrative review is limited to adults and people who had completed all cancer treatment and were disease free. However, it is noteworthy that the most desirable point for providing the lifestyle intervention is somewhat controversial. Some trials have recruited people prior to treatment for cancer from colorectal cancer screening programmes(Reference Anderson, Craigie and Caswell42), whilst others have attempted to pin point the ‘teachable moment’(Reference Park, Cho and Salner43). Moreover, now with new biological therapies people with cancer are actively treated after standard therapies to prolong survival for long periods of time, so are living with cancer(Reference Scher, Fizazi and Saad44). The only comparisons discussed are those including dietary interventions compared with control or usual care. There have been a variety of outcomes reported in dietary intervention studies and these are shown in Table 2.
Table 2. Dietary outcomes reported in the included studies
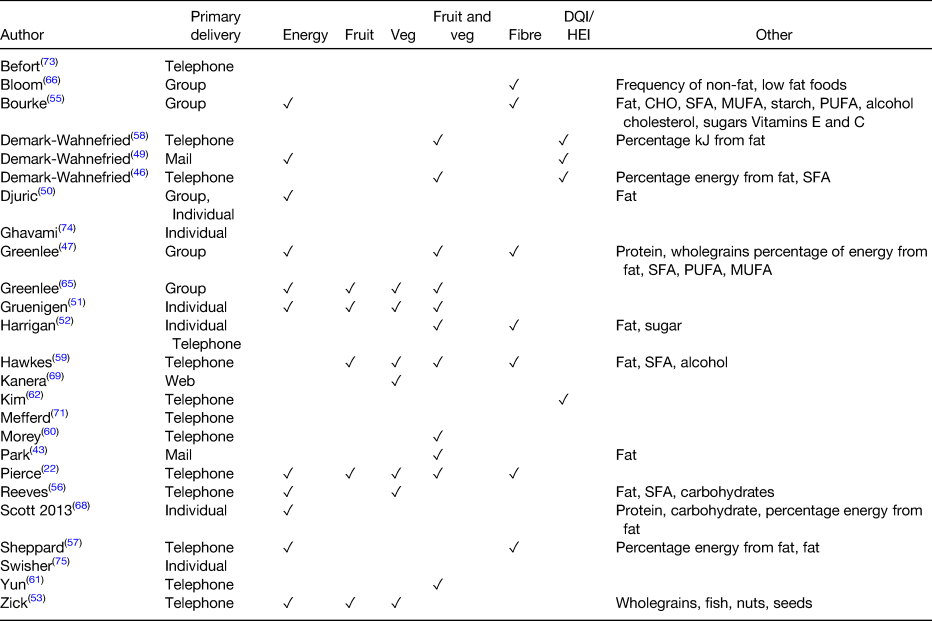
DQI, diet quality index; HEI, healthy eating index; CHO, carbohydrate.
The main mode of providing information found within RCT are telephone, mail, web-based, individual face-to-face or group sessions. The most frequently used primary delivery modes for reviewed studies are outlined in Fig. 1. However, the majority of dietary interventions described used more than one mode to deliver dietary advice. The primary intervention identified for each study was the mode assessed as being most labour intensive in relation to staffing resources or that most frequently delivered to participants. All the dietary interventions described for each study are outlined in Table 3.

Fig. 1. Modes of providing dietary advice in all comparisons.
Table 3. All modes of providing dietary intervention in the studies

*Studies provided dietary intervention but outcomes were not reported.
Behavioural change theory has been included in some of the dietary interventions used in the clinical trials undertaken to evaluate dietary interventions in people surviving cancer. The transtheoretical model with social cognitive theory was used in four studies(Reference Demark-Wahnefried, Pinto and Gritz45–Reference Morey, Sloane and Pieper48). Social cognitive theory was used in six studies, which was the most frequently reported behavioural change theory used(Reference Pierce, Natarajan and Caan22, Reference Demark-Wahnefried, Jones and Snyder49–Reference Zick, Colacino and Cornellier53). Other methods reported included acceptance and commitment theory, transtheoretical model used on its own and cognitive behavioural therapy. It is surprising that some studies conducted over the past decade failed to incorporate behavioural change theory in light of the evidence supporting the integration of psychological theory into interventions that require substantial behavioural change for individuals(Reference Michie, West and Sheals54).
Dietitians delivered the dietary interventions in seven of the studies and a mixture of other professional groups and trained personnel provided interventions in the other trials (Fig. 2). Some interventions were delivered by the internet or mailed so did not require any personnel to facilitate provision. The dietary assessment methods included in RCT with food or nutritional outcomes varied considerably between studies. The most frequently used method to provide dietary intervention was by telephone contact, whilst a few used mail, individual face-to-face, group sessions or seminars and finally a few studies used web-based interventions (Fig. 3).
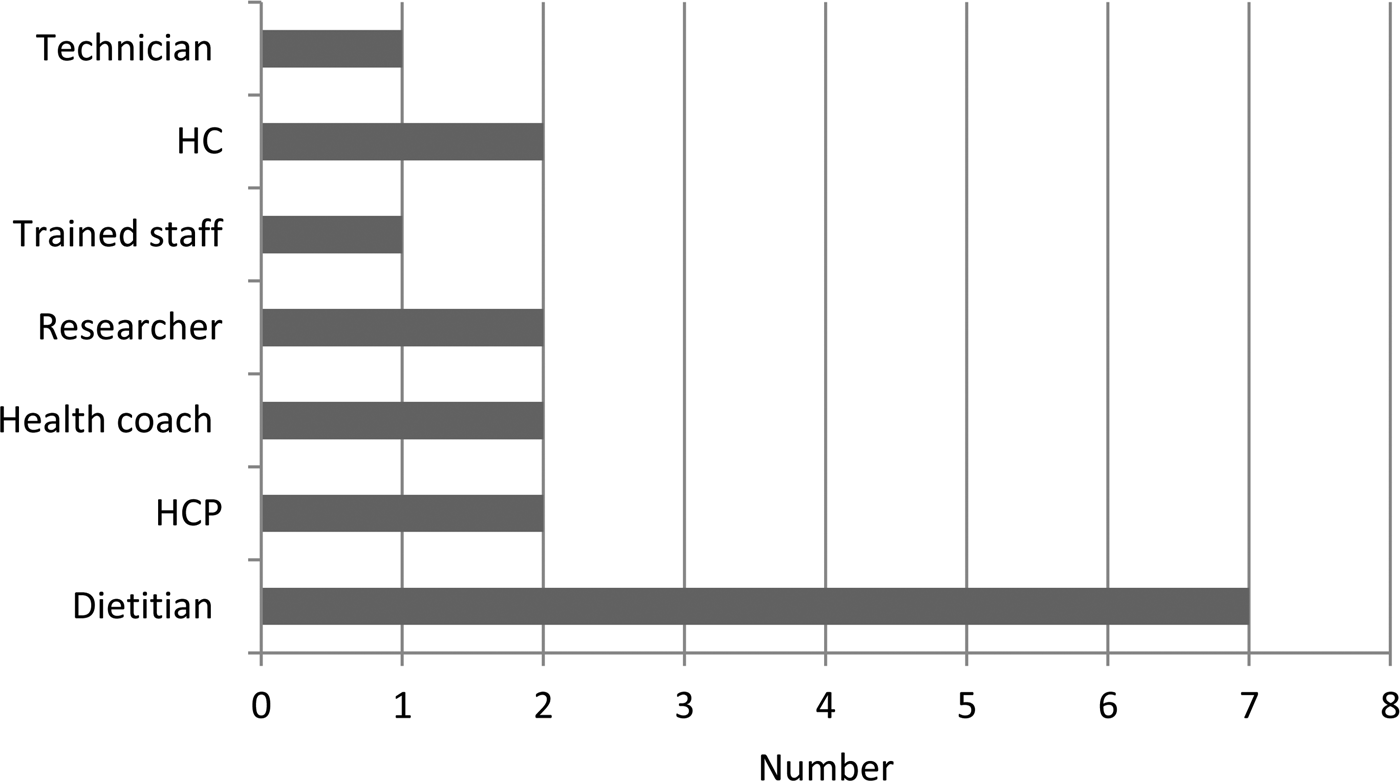
Fig. 2. Personnel providing dietary intervention in included studies. Four studies had no personnel; data from twenty three studies. HCP, health care professional; HC, health councilor.
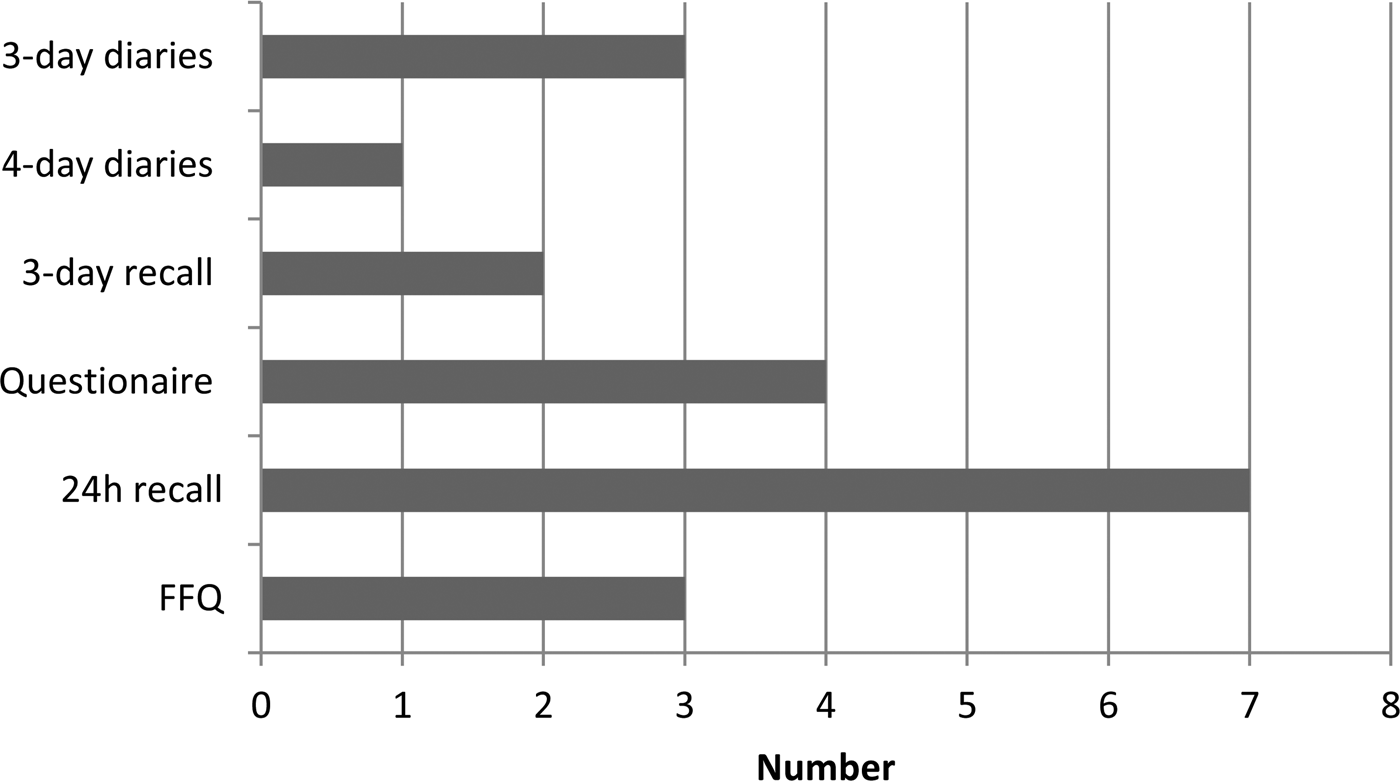
Fig. 3. Dietary assessment method used to assess food intake in the included studies.
Telephone interventions
Dietary intervention provided by telephone assessed energy intake in five studies(Reference Pierce, Natarajan and Caan22, Reference Zick, Colacino and Cornellier53, Reference Bourke, Thompson and Gibson55–Reference Sheppard, Hicks and Makambi57). Two studies reported data after 3 months and there was no difference between the intervention and control arms(Reference Zick, Colacino and Cornellier53, Reference Bourke, Thompson and Gibson55). After 6 and 12 months in one study including 3230 women after breast cancer there was again no difference between groups for energy intake(Reference Pierce, Natarajan and Caan22). One study reported a trend towards reduction of energy in the intervention group after 3 months compared with baseline in obese black women after breast cancer (mean difference −867·34 kJ, P = 0·06), albeit in a small sample size (n 22)(Reference Sheppard, Hicks and Makambi57). Evidence is showing that after cancer in studies recruiting people with difference types of cancer, there was no effect seen in the majority of studies in relation to energy intake. This finding was consistent even in a study targeting overweight or obese cancer survivors(Reference Reeves, Winkler and McCarthy56) although in one study a trend for a decrease in energy consumption was reported in obese black women who had survived breast cancer(Reference Sheppard, Hicks and Makambi57).
Fruit and vegetables servings were reported separately daily in women after breast cancer and an increase was seen at 6 and 12 months for vegetable intake, but for fruit there was no difference at 6 months but an increase after 12 months(Reference Pierce, Natarajan and Caan22). This increase in fruit and vegetables was seen in a large number of women after breast cancer in a well conducted trial, interestingly it was an intensive intervention combined with social cognitive theory(Reference Pierce, Natarajan and Caan22). Similarly, the total number of fruit and vegetable portions were reported in people after colorectal, breast and prostate cancer(Reference Demark-Wahnefried, Clipp and Morey58) and no differences were demonstrated at 6- or 12-month follow-up. Fruit and vegetable intake were reported separately in 410 participants after colorectal cancer and no differences were shown for fruit after 6 and 12 months but vegetable intake increase after 6 months, although was not sustained at 12 months(Reference Hawkes, Chambers and Pakenham59). Conversely, a study including participants with cancer at a number of sites including breast, colorectal and prostate in 641 cancer survivors measured daily servings of fruit and vegetables and showed a difference between the intervention group and control groups with older overweight long-term cancer survivors(Reference Morey, Snyder and Sloane60).
In two studies including women with breast cancer, change in fruit and vegetable intake was reported after 6 months and an increase was demonstrated in the intervention group in sixty-seven cancer survivors(Reference Harrigan, Cartmel and Loftfield52). However, the number of women who consumed more than five portions of fruit and vegetable daily(Reference Yun, Kim and Lee61) was similar within groups at all time points reported.
Four studies reported dietary fibre intake(Reference Pierce, Natarajan and Caan22, Reference Harrigan, Cartmel and Loftfield52, Reference Sheppard, Hicks and Makambi57, Reference Hawkes, Chambers and Pakenham59). One study(Reference Sheppard, Hicks and Makambi57) reported fibre intake at baseline in the intervention arm only (3 months 19·2 g sd 12·2 v. baseline 13·1 g sd 2·8, n 22). One study reported data at 6 months and 12 months(Reference Pierce, Natarajan and Caan22) and an increase in dietary fibre intake was shown at both time points in a large number of women after breast cancer.
Two studies reported data as change scores, one at 6 months(Reference Harrigan, Cartmel and Loftfield52) where an increase in fibre was shown as grams per 1000kcal in the intervention compared with the control group. Albeit, these results were refuted in another study that showed no differences in dietary fibre intake at 6- or 12-month follow-up(Reference Hawkes, Chambers and Pakenham59).
Diet quality index (DQI) was reported by two studies(Reference Demark-Wahnefried, Clipp and Morey58, Reference Kim, Shin and Lee62). These studies used different DQI scores. In one study(Reference Demark-Wahnefried, Clipp and Morey58) higher scores on the DQI indicated a better quality diet(Reference Haines, Siega-Riz and Popkin63) and data were reported at 6 months where an increase in diet quality was reported but not sustained at 12 months. In another study(Reference Kim, Shin and Lee62) the DQI was used and lower scores(Reference Patterson, Haines and Popkin64) indicated a better diet quality. The results were reported after 3 months in forty-five participants and no difference was seen.
From the studies reviewed in this narrative it was found that dietary intervention provided via the telephone did not influence energy intake. There were also inconsistencies in reporting fruit and vegetables but there was evidence form a large study that showed an improvement in fruit and vegetable intake in women after breast cancer(Reference Pierce, Natarajan and Caan22). In three studies dietary intervention provided by the telephone was found to increase dietary fibre(Reference Pierce, Natarajan and Caan22, Reference Harrigan, Cartmel and Loftfield52, Reference Sheppard, Hicks and Makambi57) although one study found contradictory results(Reference Hawkes, Chambers and Pakenham59). Some benefits were shown in diet quality at 6 months but not maintained at 12 months(Reference Demark-Wahnefried, Clipp and Morey58).
Workshops/seminars/group interventions
Five studies(Reference Greenlee, Crew and Mata47, Reference Djuric, DiLaura and Jenkins50, Reference Bourke, Thompson and Gibson55, Reference Greenlee, Gaffney and Aycinena65, Reference Bloom, Stewart and D'Onofrio66) used primarily workshops, seminars or groups to deliver dietary interventions. Four of these studies reported on energy intake. One study reported energy intake at 3 months(Reference Bourke, Thompson and Gibson55); one study reported energy at 6 months(Reference Greenlee, Crew and Mata47) and two studies after 12 months of follow-up and all showed no differences between groups(Reference Greenlee, Crew and Mata47, Reference Greenlee, Gaffney and Aycinena65). In a further study(Reference Djuric, DiLaura and Jenkins50), energy intake was reported after 12 months from a weight watchers intervention v. control but again no difference in energy intake was demonstrated, albeit this was a small study including only eighteen survivors of breast cancer.
Fruit and vegetables were reported in three of these studies. The participants increasing consumption of fruit and vegetables in both the intervention arm and the control group were very similar in one study(Reference Bloom, Stewart and D'Onofrio66). Fruit and vegetable consumption was reported by one study(Reference Greenlee, Crew and Mata47) at 6 and 12 months, with no difference demonstrated between groups(Reference Greenlee, Crew and Mata47) and these results were mirrored again with women after breast cancer(Reference Greenlee, Gaffney and Aycinena65).
Fibre was reported by two studies where group sessions were evaluated. One study(Reference Bourke, Thompson and Gibson55) reported at 3 months in eighteen survivors of colorectal cancer and there was a difference between groups although another study(Reference Greenlee, Crew and Mata47) after 6 and 12 months showed a null results.
Overall group sessions as a mode for providing a dietary intervention did not positively affect dietary intake for energy or fruit and vegetables. One small study showed a difference in dietary fibre(Reference Bourke, Thompson and Gibson55). However, the studies discussed had small sample sizes and one was a pilot study(Reference Djuric, DiLaura and Jenkins50), two studies recruited Hispanic women after breast cancer so were focusing on minority groups(Reference Greenlee, Crew and Mata47, Reference Greenlee, Gaffney and Aycinena65) and one study using groups sessions targeted young breast cancer survivors(Reference Bloom, Stewart and D'Onofrio66). It can therefore justifiably be concluded that there is a paucity of evidence evaluating group sessions in all cancer types with large representative samples.
Mailed information
In three studies information was provided by mail. One study(Reference Demark-Wahnefried, Jones and Snyder49) reported on energy intake at 6 and 12 months in forty participants and found no difference between groups at either time point.
Two studies reported on fruit and vegetable servings. One study reported data at 12 months in 519 survivors showing an increased intake of fruit and vegetables in the intervention group compared with the control group in breast and prostate survivors(Reference Demark-Wahnefried, Clipp and Lipkus46). However another study reporting fruit and vegetable intake graphically, showed the opposite after 12 months(Reference Park, Cho and Salner43). Two studies reported on diet quality and there was an improvement in diet quality in both studies favouring the dietary intervention group(Reference Demark-Wahnefried, Clipp and Lipkus46, Reference Demark-Wahnefried, Jones and Snyder49). Diet quality was assessed using DQI(Reference Haines, Siega-Riz and Popkin63).
Mailed dietary intervention did not affect energy intake although in some studies benefits were seen for fruit and vegetable intake and diet quality(Reference Demark-Wahnefried, Clipp and Lipkus46, Reference Demark-Wahnefried, Jones and Snyder49). For a relatively cost-effective means of providing a dietary intervention the results are encouraging especially for an intervention where resources would be relatively low in relation to staffing and administration. It is noteworthy that the information packs provided to participants for the mailed intervention incorporated behavioural change theory and were designed encompassing participants feedback with extensive piloting and evaluation(Reference Macri, Downs and Demark-Wahnefried67).
Individual face-to-face intervention
Four studies provided dietary intervention via individualised face-to-face consultations(Reference Djuric, DiLaura and Jenkins50–Reference Harrigan, Cartmel and Loftfield52, Reference Scott, Daley and Doll68). Energy intake was reported by one study in women after uterine cancer at 3 months(Reference von Gruenigen, Frasure and Kavanagh51) and 12 months and there was no difference seen after each time point. Another study also reported there was no difference between groups for energy intake but no data were presented(Reference Scott, Daley and Doll68). Energy intake was reported in a small sample and again there was no real difference between the individualised groups v. control(Reference Djuric, DiLaura and Jenkins50). Fruit and vegetable servings were reported by one study(Reference von Gruenigen, Frasure and Kavanagh51) after 12 months and no difference was shown, although in another study that reported change scores there was a difference between groups favouring the intervention group(Reference Harrigan, Cartmel and Loftfield52). In this same study a difference in dietary fibre intake was seen after 6 months. In summary face-to-face dietary intervention improved fruit and vegetable intake in one study(Reference Harrigan, Cartmel and Loftfield52) but not in another(Reference von Gruenigen, Frasure and Kavanagh51) and dietary fibre(Reference Harrigan, Cartmel and Loftfield52) but none of the studies affected energy intake.
Web based
One study(Reference Kanera, Willems and Bolman69) provided dietary intervention via the internet and recorded vegetable consumption as an outcome at 6 and 12 months and no differences were demonstrated at either time point. In summary there is no evidence that a web-based intervention can improve fruit and vegetable intake.
Discussion
There is clear evidence supporting the link between cancer and dietary intake from a substantial amount of epidemiological evidence(16, Reference Bradbury, Appleby and Key20). People living after cancer have an increased level of motivation for change(Reference Demark-Wahnefried, Aziz and Rowland11), which is well documented with some people actively engaging in eating a healthier diet and following a healthier lifestyle after a diagnosis(Reference Burden, Stamataki and Hill36). This is supported within psychosocial research investigating motivation levels, intention and willingness to change, after a major life event where people reprioritise the importance of healthy behaviours in their lives(Reference Stanton, Rowland and Ganz9). People's lived experiences from qualitative interviews have supported these findings not only in colorectal cancer but in other cancer types(Reference Burden, Stamataki and Hill36, Reference Williams and Jeanetta37). It is therefore a logical next step from the evidence base to investigate ways to capitalise on increased awareness of healthier lifestyle initiatives after a diagnosis of cancer. There is evidence that cancer survivors use health care services more than their age matched peers(Reference Søgaard, Thomsen and Bossen26). However, there is no evidence that dietary interventions can reduce morbidity and mortality over a period of 10 years in people after cancer(Reference Pierce, Natarajan and Caan22, Reference Søgaard, Thomsen and Bossen26), although a decade may not be long enough for a dietary intervention to effect these outcomes. Albeit, in women who were given the intervention whilst still receiving treatment for breast cancer benefits of dietary intervention were demonstrated in a large trial in the USA in relation to recurrence free survival and disease free survival(Reference Chlebowski, Blackburn and Thomson70). It is difficult to conduct studies for long periods of time over a life course due to the difficulties of obtaining funding for long-term follow-up. Participants, who are lost to follow-up in RCT may be those that have higher rates of comorbidities and who are less committed to the intervention, introducing some degree of bias.
From studies reviewed the most favoured method of providing dietary interventions was via the telephone(Reference Demark-Wahnefried, Clipp and Morey58, Reference Hawkes, Chambers and Pakenham59, Reference Morey, Snyder and Sloane60, Reference Kim, Shin and Lee62, Reference Mefferd, Nichols and Pakiz71), and this was often combined with written materials(Reference Demark-Wahnefried, Clipp and Lipkus46) or lifestyle coaching(Reference Pierce, Natarajan and Caan22). Overall there was evidence that telephone-based dietary interventions increased fruit and vegetables intake primarily in breast cancer(Reference Pierce, Natarajan and Caan22) and at some time points in colorectal cancer and mixed cancer groups(Reference Hawkes, Chambers and Pakenham59), although the benefits were not consistent across all the studies(Reference Reeves, Winkler and McCarthy56, Reference Yun, Kim and Lee61). Dietary interventions were also shown to increase dietary fibre intake in women after breast cancer(Reference Pierce, Natarajan and Caan22), although again this increase was not seen consistently(Reference Hawkes, Chambers and Pakenham59). Diet quality also improved with telephone dietary intervention in one study with a mixed cancer cohort at 6 months but not 12 months(Reference Demark-Wahnefried, Clipp and Morey58) although this was not repeated in a study with breast cancer survivors using a different scale(Reference Kim, Shin and Lee62).
Dietary intervention provided in groups did not change energy intake(Reference Greenlee, Crew and Mata47, Reference Djuric, DiLaura and Jenkins50, Reference Bourke, Thompson and Gibson55, Reference Greenlee, Gaffney and Aycinena65), nor fruit and vegetable intake overall as most studies did not report any increase(Reference Greenlee, Crew and Mata47, Reference Greenlee, Gaffney and Aycinena65). Studies evaluating dietary interventions provided by mail only reported on a few dietary outcomes and for fruit and vegetable intake there were contradictory results between two studies(Reference Park, Cho and Salner43, Reference Demark-Wahnefried, Clipp and Lipkus46); however, diet quality in two mixed cancer cohorts was shown to improve(Reference Demark-Wahnefried, Clipp and Lipkus46, Reference Demark-Wahnefried, Jones and Snyder49).
Dietary intervention provided by face-to-face consultations demonstrated a difference for fruit and vegetables and dietary fibre in women after breast cancer(Reference Harrigan, Cartmel and Loftfield52) but not after uterine cancer(Reference von Gruenigen, Frasure and Kavanagh51). Again there was no change in energy intake(Reference Djuric, DiLaura and Jenkins50–Reference Harrigan, Cartmel and Loftfield52). The evaluation of web-based dietary intervention was limited and did not lead to any positive dietary modifications(Reference Kanera, Willems and Bolman69). The development of dietary interventions designed to be delivered via the internet is limited in cancer survivorship so this may be an area for future developments with the advent of advancing technologies leading to more interactive, user friendly packages.
There was limited success across all studies to modify energy intake. This is not really surprising as the dietary interventions were not necessarily focusing on reducing energy and predominantly were providing healthy eating dietary advice.
We present an overview of current RCT evaluating dietary interventions in people who have survived cancer. A limiting factor in the discussion is the absence of quality assessment using a recognised tool to determine the robustness of the evidence base. Some studies were subject to type two error due to small sample sizes and thus imprecision, whilst other studies had high risks to bias due to absence of blinding, lack of objective outcomes and high levels of attrition. The dietary outcomes reported were also limited in studies and all used a wide variety of dietary assessment methods to measure nutrients or food intake.
In conclusion, there is a lack of robust evidence that energy, fruit and vegetable intake, dietary fibre or overall diet quality can be improved in people who have survived cancer despite ample evidence linking a poor diet to cancer occurrence from population based studies(16). None of the approaches to change diet, be they individual face-to-face, group, telephone, mail or internet based showed strong long-term effects on dietary variables other than the large studies with women with breast cancer(Reference Pierce, Natarajan and Caan22). Whilst this is disappointing it is not surprising given the difficulty of assessing habitual dietary intake and embedding the principles of behaviour change theory(Reference Michie, Richardson and Johnston72) into a long-term dietary intervention.
Financial Support
Dr Sorrel Burden was funded by a National Institute of Research Senior Clinical Lectureship.
Conflict of Interest
None.








