Published online by Cambridge University Press: 21 January 2014
Clotrimazole (C22H17ClN2, 1-[(2-chlorophenyl)(diphenyl)methyl]-1H-imidazole) and fluconazole (C13H12F2N6, 2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazole-1-yl)propan-2-ol) are two active pharmaceutical ingredients commonly used in the treatment and prevention of superficial and systemic fungal infections. The X-ray powder diffraction data and the unit cell parameters of anhydrous clotrimazole [Triclinic, P 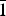 ${\bar 1}$ , a = 8.776(1) Å, b = 10.571(2) Å, c = 10.622(3) Å, α = 114.08(2)°, β = 96.87(2)°, γ = 97.61(2)°, V = 875.2(2) Å3, Z = 2] and monohydrated fluconazole [Triclinic, P
${\bar 1}$ , a = 8.776(1) Å, b = 10.571(2) Å, c = 10.622(3) Å, α = 114.08(2)°, β = 96.87(2)°, γ = 97.61(2)°, V = 875.2(2) Å3, Z = 2] and monohydrated fluconazole [Triclinic, P 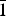 ${\bar 1}$ , a = 5.6353(4) Å, b = 11.753(1) Å, c = 12.326(1) Å, α = 71.220(8)°, β = 79.896(9)°, γ = 84.35(1)°, V = 760.13(9) Å3, Z = 2] are reported.
${\bar 1}$ , a = 5.6353(4) Å, b = 11.753(1) Å, c = 12.326(1) Å, α = 71.220(8)°, β = 79.896(9)°, γ = 84.35(1)°, V = 760.13(9) Å3, Z = 2] are reported.