Introduction
In India yellow sarson (Brassica rapa var yellow sarson) is an important oilseed crop in India and is valued for its oil-rich seeds (Sood and Kumari, Reference Sood and Kumari2019); with more oil content than other species of the rapeseed mustard group, yellow sarson becomes an important crop. The present status of rapeseed mustard as the third most important source of vegetable oils is attributable to the success of plant breeders in developing low glucosinolates and zero erucic acid varieties. Quality-wise the improved rapeseed oil is now equivalent to peanut and olive oil. From the nutritional, cooking and stability points of view, possibilities exist for further alterations in the fatty acid composition in favour of increased levels of oleic and linoleic acids simultaneously with a reduction in linolenic acid content (Ahuja and Banga, Reference Ahuja, Banga, Ahuja and SK1993). Yellow sarson exhibits a beautiful flower colour and diverse floral parts, ranging from petalous to apetalous flowers. It is predominantly found in the petalous form, with some mutants displaying the apetalous trait. Yellow sarson presents a captivating subject for research, specifically focused on unravelling the inheritance patterns associated with the petalous and apetalous traits in this plant species (Rahman, Reference Rahman2001). The petalous trait, marked by vibrant yellow petals, is noteworthy due to its pivotal role in attracting pollinators, particularly bees (Jager et al., Reference Jager, Willis-Jones, Critchley and Glover2017). This attraction facilitates effective cross-pollination, thereby enhancing seed production and the potential for increased yield. Conversely, the apetalous trait, characterized by the absence of petals, has its own set of merits in specific agricultural contexts (Evans et al., Reference Evans, Gemmill, Werner and Williams2003). The apetalous character in rapeseed has been studied for two reasons. First, a physiological potential advantage has been claimed. Petals cause a reduction in reflection of photosynthetically active radiations (PAR) so that apetalous lines transmit 34% of the incident PAR to the photosynthetic tissues versus 17% in the petalous ones. Moreover, on the main stem, leaves of apetalous plants persist longer and pod production and fertility are higher than in the near isogenic conventional lines. Second, lines without petals may reduce sclerotinia (Sclerotinia sclerotiorum) disease transmitted to healthy tissue by petals contaminated with this pathogen, the lack of petals simplifies the harvesting process, mitigating concerns related to petal contamination in harvested seeds (Baltzer et al., Reference Baltzer, Hewlin, Reekie, Taylor and Boates2002). Apetalous genotypes within Brassica species exhibit diverse genetic origins. The majority of these genotypes were either serendipitously discovered through spontaneous mutations (Singh, Reference Singh1961a, Reference Singh1961b; Buzza, Reference Buzza1983; Lu and Fu, Reference Lu and Fu1990) or emerged as by-products of other research endeavours (Malik et al., Reference Malik, Vyas, Ranggaswamy and Shivanna1999). The inheritance patterns of the apetalous trait vary across different sources. Investigations into genotypes with apetalous flowers are most extensively conducted in Brassica napus. Depending on the specific origin of the apetalous trait, it is regulated by either two recessive genes (Buzza, Reference Buzza1983), four recessive genes (Lu and Fu, Reference Lu and Fu1990), an epistatic interaction involving recessive alleles at a pair of homologous loci or interactions between alleles at three loci (Kelly et al., Reference Kelly, Fray, Arthur and Lydiate1995). Regarding B. rapa var yellow sarson crops, very scant studies are conducted for petalous and apetalous trait inheritance. Previous studies on B. rapa reveal the fact that the trait is controlled by one single recessive gene (Singh, Reference Singh1961a, Reference Singh1961b; Cours and Williams, Reference Cours and Williams1977). To date, no research has been conducted over due course of time. The benefits of apetalous traits also drive us in the direction of studying the inheritance pattern of the genes involved in petalous and apetalous traits so that we can incorporate the trait in our hybrid varieties. By conducting segregation and chi-squared analyses on an unprecedented F2 population exhibiting petal loss derived from a novel yellow sarson cross, we aim to pinpoint loci controlling the apetalous trait in this crop. Achieving this objective will fill a critical knowledge gap inhibiting targeted breeding efforts. More broadly, it will provide fundamental insight into the context-specific genetic regulation of floral morphology with transferability across Brassica oilseed crops. Our findings will aid preservation of genetic diversity important for global food security in a changing climate.
Materials and methods
Plant materials
Experiments were conducted at the Crop Research Center, GBPUAT, Pantnagar, India using three yellow sarson crosses: (1) Pant Sweta (petalous) × apetalous, (2) Pant Girija (petalous) × apetalous and (3) YSH0401 (petalous) × apetalous. The petalous parents were selected from the university's germplasm collection. F1, F2, BC1P1 and BC2P2 generations were developed for each cross through controlled pollinations from 2019 to 2021.
Phenotypic evaluation
The petalous trait (yellow petals present) and apetalous trait (no petals) were visually scored in a binary qualitative manner in the parents and different generations (F1, F2, BC1P1 and BC2P2). Numbers of plants exhibiting each phenotype were recorded.
Statistical analysis
Segregation ratios were analysed using chi-squared tests to check for goodness-of-fit between observed phenotypic ratios and expected genetic ratios. The chi-squared (χ 2) formula was as below:
where the summation is overall phenotypic classes.
For monogenic inheritance, the expected phenotypic ratios were based on the segregation of a single gene with two alleles (A and a) exhibiting complete dominance. The expected ratios were,F2 generation: 3 petalous:1 apetalousBC1 generation: 1 petalous:1 apetalous
For digenic inheritance, the expected ratios were based on the segregation of two independently assorting genes. Several possible two-gene models with varying degrees of dominance were tested. The χ 2 statistics was calculated for each generation and genetic model. This was compared to critical values from the χ 2 distribution at a α significance level of 0.05 to evaluate if the deviations between observed and expected ratios were significant. Significant deviations indicate that the observed inheritance does not follow the proposed genetic model.
Results
Inheritance pattern of petalous trait in Pant Sweta × apetalous cross
From Table 1, it is clear that in the F1 generation, 20 plants were evaluated. All 20 F1 plants exhibited petalous flowers, yielding a petalous:apetalous ratio of 1:0. In the F2 population consisting of 230 plants, we observed 163 petalous and 67 apetalous individuals. This equates to a petalous:apetalous ratio of 3:1 which aligns with the expectation under monogenic inheritance with dominance of the petalous trait. The χ 2 value was calculated to be 1.86, indicating no significant deviation between the expected and observed ratios.
Table 1. Inheritance pattern of petalous condition in different generation of cross Pant Sweta (petalous) × apetalous

The backcross with the petalous parent Pant Sweta (BC1P1) included 320 plants, all of which displayed petalous flowers. The 1:0 petalous:apetalous ratio mirrors the anticipated ratio given the dominance of petalous trait. A chi-squared test showed no significant difference between the observed and expected ratios (χ 2 = 0.00). The backcross with the apetalous parent (BC2P2) consisted of 310 plants. We recorded 160 petalous and 150 apetalous progeny, precisely matching the expected 1:1 ratio under monogenic inheritance. This was further supported by a χ 2 value of 0.32.
Inheritance pattern of petalous trait in Pant Girija × apetalous cross
Table 2 and Fig. 1 provide a clear picture about plants in F1, F2, BC1P1 and BC2P2. The F1 generation comprised of 25 plants, all exhibiting petalous flowers. The 1:0 petalous:apetalous ratio agrees with the prediction under monogenic inheritance with dominance of the petalous trait. In the F2 population of 250 plants, 163 petalous and 87 apetalous individuals were observed more clearly in Fig. 1, reflecting a 3:1 ratio as expected by the monogenic model. The χ 2 value was 1.52 (P > 0.05), confirming no significant deviation from the hypothesized 3:1 ratio.

Figure 1. Segregation pattern in Pant girija × apetalous cross.
Table 2. Inheritance pattern of petalous condition in different generation of cross Pant Girija (petalous) × apetalous

The backcross with petalous parent Pant Girija (BC1P1) contained 340 progeny, all of which displayed petalous flowers. The 1:0 petalous:apetalous ratio aligns precisely with the prediction given petalous trait dominance. A chi-squared test showed no difference between the observed and expected ratios (χ 2 = 0.0).
The backcross with the apetalous parent (BC2P2) consisted of 320 plants. We recorded 170 petalous and 150 apetalous individuals, accurately matching the postulated 1:1 ratio under monogenic inheritance. The χ 2 value was 0.38 (P > 0.05), substantiating the lack of deviation between the observed and expected.
Inheritance pattern of petalous trait in YSH0401 × apetalous cross
Table 3 and Fig. 2 show us that the F1 generation consisted of 30 plants, all exhibiting petalous flowers and confirming the expected 1:0 petalous:apetalous ratio under monogenic inheritance with dominance of the petalous trait. In the F2 population of 260 plants, we recorded 170 petalous and 90 apetalous individuals, reflecting a ratio of 3:1 petalous:apetalous as predicted by the inheritance model. The χ 2 statistic was 1.80 (P > 0.05), substantiating no significant deviation from the 3:1 expectation.
Table 3. Inheritance pattern of petalous condition in different generation of cross YSH0401 (petalous) × apetalous
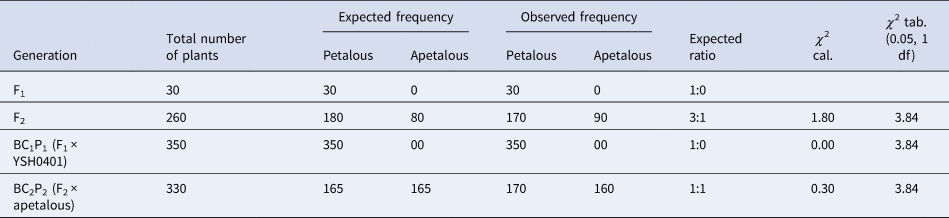

Figure 2. Petalous and apetalous plant numbers in different generations.
The backcross with petalous parent YSH0401 (BC1P1) contained 350 progeny, all petalous. The 1:0 petalous:apetalous ratio precisely matches predictions given the dominant petalous phenotype. A chi-squared test validated the lack of difference between observed and expected (χ 2 = 0.00).
For the backcross with the apetalous parent (BC2P2), among 330 plants, we observed 170 petalous and 160 apetalous individuals. This 1:1 ratio again aligns accurately with assumptions under monogenic inheritance. The χ 2 statistic of 0.30 (P > 0.05) further confirms that the observed numbers match the expected values.
The results endorse the proposed monogenic, dominance inheritance pattern for petalous trait in the YSH0401 × apetalous Brassica cross, with complete dominance over the apetalous phenotype.
Crosses and their seed yield
From Table 4, it is clear that all three crosses below had more yield in petalous genotypes than in apetalous genotypes.
Table 4. Various generations and crosses and their seed yield
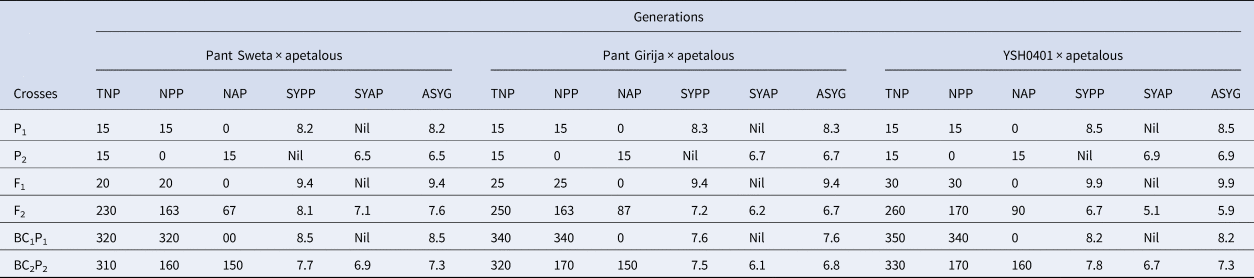
TNP, NPP, NAP, SYPP, SYAP and ASYG refer to total number of plants; number of petalous plants; number of apetalous plants; seed yield per plant of petalous type plants; seed yield per plant of apetalous type plants and average yield of generation.
Pant Sweta × apetalous cross:
• Petalous plants achieved the highest seed yields in the F1 generation at 9.4 g/plant.
• In the segregating F2 generation, petalous plants averaged 8.1 g/plant, while apetalous averaged 7.1 g/plant. Petalous significantly outpaced apetalous plants by 1.2 times.
• In the backcross BC2P2, the seed yield gap between petalous at 7.7 g/plant and apetalous at 6.9 g/plant was at 1.1×.
Pant Girija × apetalous cross:
• Petalous and apetalous plant effects can be compared from the F2 and backcross generations. In the F2, petalous plants had a seed yield of 7.2 g/plant versus 6.2 g/plant for apetalous.
• This 1.2× yield advantage for petalous over apetalous also persisted in the BC2P2 generation with petalous at 7.5 g/plant and apetalous at 6.1 g/plant, a 1.2× ratio.
• Thus across all generations, petalous plants significantly out-yielded apetalous plants by around 20%.
YSH0401 × apetalous cross:
• The highest seed yields were recorded in the F1 at 9.9 g/plant with all petalous plants.
• In the F2 generation, yields of 6.7 g/plant for petalous plants and 5.1 g/plant for apetalous plants were observed, a moderately high 1.3× yield gap.
• This gap in the backcross BC2P2 generation to 7.8 g/plant for petalous plants and 6.7 g/plant for apetalous plants, was consistent
• Overall, the benefit of petalous plants for seed yield relative to apetalous plants was consistent across generations.
Seed yields were superior for petalous plants across all crosses and generations, with the relative yield advantage ranging from 1.1× to 1.3× over the corresponding apetalous plants.
Discussion
A comprehensive investigation into the inheritance pattern of the petalous condition in various Brassica plants has provided valuable insights into the genetic dynamics of this trait. The consistent observation of complete dominance of the petalous trait in the F1 generations across different crosses aligns with classical Mendelian genetics, establishing a solid foundation for understanding the genetic control of this important characteristic (Stern, Reference Stern1970).
The anticipation of a 3:1 ratio in the F2 generation further confirmed the dominance of the petalous allele, offering practical implications for plant breeders. The ease of obtaining petalous plants due to the dominant nature of the trait simplifies the selection process. However, the persistence of the petalous trait in backcross generations (BC1P1 and BC2P2) emphasizes the challenges associated with incorporating the apetalous trait into Brassica varieties. The need for six to seven generations of backcrossing to achieve a desirable apetalous genotype underscores the stability and dominance of the petalous allele (Chapman et al., Reference Chapman, Daniels and Scarisbrick1984).
The chi-squared analysis supports the adherence to an expected 1:1 ratio in the backcross generations, providing statistical validation for the observed dominance pattern (Huang et al., Reference Huang, Liang, Lu and Chen2006). This concordance between the expected and observed ratios enhances the robustness of the study's findings and contributes to the reliability of the identified genetic patterns.
The implications of these findings extend beyond the immediate understanding of petalous inheritance in Brassica plants. Plant breeders can leverage this knowledge to make informed decisions in selecting and developing plant lines with specific trait expressions. The dominance pattern observed in this study guides breeding efforts aimed at improving Brassica varieties with the desired petalous characteristics. This, in turn, contributes to a broader goal of advancing crop improvement and enhancing agricultural sustainability.
In advancing the knowledge of this subject, future research could delve deeper into the molecular mechanisms underlying the dominance of the petalous allele. Investigating specific genes and regulatory elements associated with petal development could provide a more nuanced understanding of the trait inheritance. Additionally, exploring the environmental factors that may influence the expression of the petalous condition could contribute to a more comprehensive understanding of the trait's plasticity.
Furthermore, the study opens avenues for the exploration of marker-assisted selection techniques to expedite the breeding process. Identifying molecular markers linked to the petalous trait can facilitate more efficient and targeted breeding strategies, reducing the time and resources required to develop Brassica varieties with specific petalous characteristics.
In conclusion, the present study significantly advances our understanding of the genetic inheritance of the petalous trait in Brassica plants. The observed dominance pattern, supported by statistical analyses, provides a practical framework for plant breeders to manipulate and improve petalous characteristics. As we move forward, molecular investigations and the integration of advanced breeding techniques will likely enhance our ability to tailor Brassica varieties to meet evolving agricultural needs.
Acknowledgements
The authors thank all the personnel working at the Crop Research Center, G.B. Pant University of Agriculture and Technology (GBPUAT), Pantnagar, Uttarakhand, India. We also appreciate all the help provided by the Department of Genetics and Plant Breeding, G.B. Pant University of Agriculture and Technology (GBPUAT), Pantnagar, Uttarakhand, India.
Competing interests
None.








