Introduction
The maintenance of seed viability during ex situ storage in genebanks is a key factor in the conservation of plant genetic resources. Most plant species, especially those that are crop related, have desiccation-tolerant seeds that may survive for several years to several decades. Seed longevity may vary among species and genotypes, but eventually the viability of a seed lot declines to an unacceptable level, which necessitates the production of next-generation seeds (Walters et al., Reference Walters, Wheeler and Grotenhuis2005; Nagel et al., Reference Nagel, Vogel, Landjeva, Buck-Sorlin, Lohwasser, Scholz and Börner2009; van Treuren et al., Reference van Treuren, de Groot and van Hintum2013). However, regeneration is a costly genebank operation and may also negatively affect the genetic integrity of an accession through exposure to the potential influence of genetic drift, selection, contamination and human error. Therefore, seeds should be stored under conditions that maximize their longevity while keeping storage costs and logistics to a manageable level (Engels and Visser, Reference Engels and Visser2003). Traditionally, low seed moisture content and storage temperature are the key conditions considered for the ex situ conservation of desiccation-tolerant seeds. The role of oxygen during storage has so far received little attention from the genebank community, although its detrimental effects on seed viability have been demonstrated in several studies (Gane, Reference Gane1948; Roberts and Abdalla, Reference Roberts and Abdalla1968; Shejbal, Reference Shejbal1979; Bennici et al., Reference Bennici, Bitonti, Floris, Gennai and Innocenti1984; Shrestha et al., Reference Shrestha, Shepherd and Turnbull1985; Barzali et al., Reference Barzali, Lohwasser, Niedzielski and Börner2005; González-Benito et al., Reference González-Benito, Pérez-García, Tejeda and Gomez-Campo2011; Schwember and Bradford, Reference Schwember and Bradford2011; Groot et al., Reference Groot, Surki, de Vos and Kodde2012).
Recently, the standards for genebank management procedures have been revised at the request of the Commission on Genetic Resources for Food and Agriculture (CGRFA, 2013) based on current technological and scientific knowledge. Standards for drying and storage aimed at maintaining seed viability have been formulated only in terms of seed moisture content and storage temperature. The revised genebank standards do include the use of hermetically closed containers for the long-term storage of orthodox seeds, but this recommendation addresses preventing moisture uptake by the seeds instead of also creating anoxic conditions. Genebanks are using different types of containers, such as sealed laminated aluminium foil bags, sealed metal cans, glass jars with rubber sealing, glass jars with plastic screw caps or even plastic or paper containers for temporary storage. These seed storage containers may differ in the level of permeability for oxygen, while some impermeable ones may enclose relatively large amounts of oxygen (Fig. 1).

Fig. 1 Genebank seed storage in glass jars with rubber sealing, showing relatively large ratios of enclosed oxygen to the number of stored seeds.
Previous studies using seeds of lettuce, cabbage, soybean and barley have demonstrated deleterious effects on seed viability under high oxygen concentrations (Groot et al., Reference Groot, Surki, de Vos and Kodde2012). Herein, we report on follow-up studies carried out to investigate the permeability for oxygen and water vapour of various types of seed storage containers, the oxygen uptake by dry lettuce seeds stored in closed containers at 20°C and the effect of anoxia on seed viability during the storage of primed celery and celeriac seeds. Primed celery and celeriac seeds are reputed for their short storability and, consequently, are ideal study materials for testing seed longevity in a short-term experiment. Implications of the results for the optimization of seed storage conditions in genebanks are discussed.
Materials and methods
Determination of the permeability of seed storage containers
Six types of glass storage containers and closures were tested for their permeability for water vapour and oxygen (Fig. 2). Comparable containers have previously been used by Gómez-Campo (Reference Gómez-Campo2002) for seed storage research and shown to be impermeable or highly impermeable to water vapour. Kilner jars were purchased from a local hardware store (Wageningen, the Netherlands), DURAN® borosilicate glass laboratory bottles from the DURAN Group GmbH (Mainz, Germany) and jam jars from Flaschenland (Sessenhausen, Germany). The plastisol that is used for the hermetic closure of jam jars is a suspension of polyvinyl chloride particles in a plasticizer. Glass vessels, such as those used by the Millennium Seed Bank Project, were kindly provided by the Royal Botanic Gardens, Kew (London, UK).
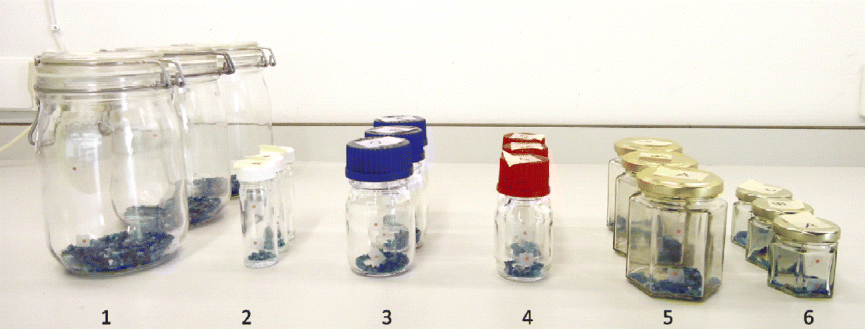
Fig. 2 Glass seed storage containers with different closures that were tested for their permeability for water vapour and oxygen: Kilner jars with glass lid and rubber sealing (1), glass vessels with a white polypropylene screw cap (2), DURAN® borosilicate glass laboratory bottles with either blue polypropylene screw caps (3) or red polybutylene terephthalate screw caps (4), and 198 ml (5) and 129 ml (6) jam jars with a metal twist-off lid lined on the inner side with a layer of flexible plastisol.
The permeability of the containers and closures for water vapour was tested by inclusion of regenerated silica gel with a blue cobalt chloride colour indicator. To investigate the permeability for oxygen, two pink optical oxygen sensor dots (PreSens – Precision Sensing GmbH, Regensburg, Germany) were attached to the inside of each transparent container before flushing the container with nitrogen gas and closing it. Potential colour changes of the silica gel and the oxygen sensor dots were monitored for a period of at least 3 months.
Additionally, the permeability of laminated aluminium foil bags that are used for the vacuum storage of seeds of, among others, the collections of the Centre for Genetic Resources, the Netherlands (CGN), and those managed by the United States Department of Agriculture (Walters et al., Reference Walters, Wheeler and Grotenhuis2005; van Treuren et al., Reference van Treuren, de Groot and van Hintum2013) was also tested. These bags consist of thin layers of aluminium foil, polyethylene and polyester. The lack of transparency of the seed bags did not allow the use of optical indicators and sensors as used for the glass seed containers. Permeability tests were carried out on seed samples from 50 barley and 50 wheat accessions of the CGN. The vacuum-sealed seed bags had been stored at 4°C for at least 20 years. Before packaging and storage, the samples had been equilibrated in a drying room at 15% relative humidity (RH) and 15°C. After retrieval from the 4°C storage facility, the samples were allowed to adjust to room temperature overnight before opening. To test the permeability for oxygen, the vacuum of the seed bags was checked, while the permeability for water vapour was examined by comparing the seed weight directly after opening of the bags with the seed weight after re-equilibration at 15% RH and 15°C for 1 week.
Measurement of oxygen uptake by seeds
A large batch of lettuce seeds was provided by the seed company Rijk Zwaan (de Lier, the Netherlands). To obtain seeds with a fixed low water content, the seeds were equilibrated in a cabinet with circulating air above a saturated calcium chloride solution. Subsequently, the seeds and a RH data logger (EasyLog EL-USB-2; Lascar Electronics Ltd, Wiltshire, UK) were placed in a closed 1-litre Kilner jar. An equilibrium RH value of 39% was recorded after 1 week of storage at 20°C. Two replicate samples of 10 g seeds were placed in glass jam jars with an internal volume of 47 ml. The volume of 10 g lettuce seeds, determined by water displacement in a graduated cylinder, was 18 ml. Two optical oxygen sensor dots were placed in the glass jar to analyse changes in oxygen concentrations during storage at 20°C in the dark for a period of 450 d. Both dots were measured three times per analysis and an average of these six measurements per jar was calculated.
Evaluation of the effects of anoxia on seed viability
The effects of anoxia on seed viability were tested on (non-commercial) primed and pelleted celery and celeriac seeds provided by Rijk Zwaan. Celery and celeriac are different crop types of the species Apium graveolens. In the case of non-treated seeds of these crop types, germination takes several weeks during which the embryo grows. To obtain seeds with fast and uniform germination, the seed company had performed a moist priming treatment that allows germination processes to progress, but without radicle protrusion. The seeds were pelleted after priming and drying to facilitate mechanical sowing.
Primed and pelleted celery and celeriac seeds were stored in 129 ml jam jars with, or without, an Ageless® ZPT oxygen absorber with a capacity of 200 ml oxygen. The oxygen absorbers were donated by Mitsubishi Gas Chemical Company, Inc. (Tokyo, Japan). In addition to iron powder, these oxygen absorbers also contain a moisturizing agent to increase the rate of iron oxidation, hence the rate of oxygen depletion. Therefore, we also tested the effect of adding 5 g zeolite seed Drying Beads® to the seed containers (Hay et al., Reference Hay, Thavong, Taridno and Timple2012). These seed Drying Beads® were donated by Rhino Research (Phichit, Thailand). In an accompanying experiment with 198 ml glass jam jars containing an Ageless® ZPT oxygen absorber and a RH data logger (hence the larger container volume), the RH was found to increase to 88% within 2 d, whereas additional inclusion of zeolite seed Drying Beads® resulted in 0% RH.
The effects of the Ageless® ZPT oxygen absorber and the zeolite seed Drying Beads® were tested at 35°C. For comparison, similar jars with primed and pelleted celery or celeriac seeds but without an oxygen absorber and zeolite seed Drying Beads® were stored at − 20°C. After 17 d of storage, all the jars were adjusted to room temperature, after which they were opened and left overnight to allow potential ultra-dry seeds to humidify. Germination was tested for three replicates of 50 seeds using a Jacobsen table, conditioned at 20°C and 12 h of light. The final frequency of normal seedlings was evaluated 18 d after imbibition.
Results
Permeability of seed storage containers
During the 3-month experiment, the silica gel remained blue in all the test containers (Fig. 2), indicating a high level of impermeability for water vapour. However, an increase in internal oxygen concentrations was observed in the glass laboratory bottles closed with either blue polypropylene or red polybutylene terephthalate screw caps, although considerable variation was observed between the replicates for each of these two types of closures. Similar results were obtained for the ‘Millennium Seed Bank’ glass vessels closed with white polypropylene screw caps. Oxygen levels in the Kilner and jam jars maintained their initial value. Apparently, adequate sealing is provided by the flexible rubber ring pressed between the Kilner jar and the lid and by the flexible plastisol layer pressed between the jam jar and the twist-off lid.
All vacuum-sealed laminated aluminium foil bags containing wheat or barley seeds had maintained their vacuum during storage for at least 20 years, exhibiting impermeability for atmospheric gases. Seed weight before and after re-equilibration at 15% RH and 15°C appeared to be highly correlated (Fig. 3), indicating that during storage in laminated aluminium foil bags no exchange of moisture between the environment and seeds had occurred.
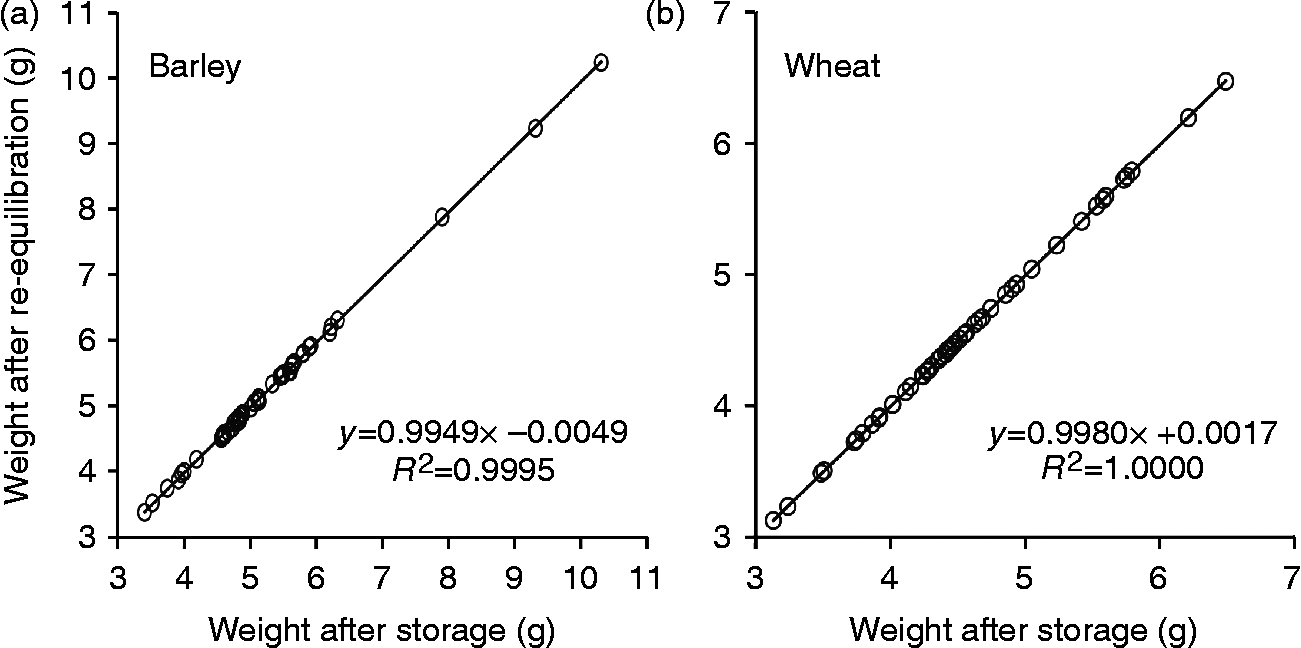
Fig. 3 Seed weight of 50 barley (a) and 50 wheat (b) samples before and after re-equilibration at 15°C and 15% relative humidity (RH). The examined seed samples had been stored in laminated aluminium foil bags at 4°C for at least 20 years after drying at 15°C and 15% RH.
Oxygen uptake by seeds
Oxygen levels within hermetically closed jam jars containing dry lettuce seeds dropped to approximately one-third of the initial value within 1 year of storage (Fig. 4). The oxygen uptake by the seeds followed a negative exponential rather than a linear trend in time, indicating that the rate of oxygen uptake declined during the course of the experiment.
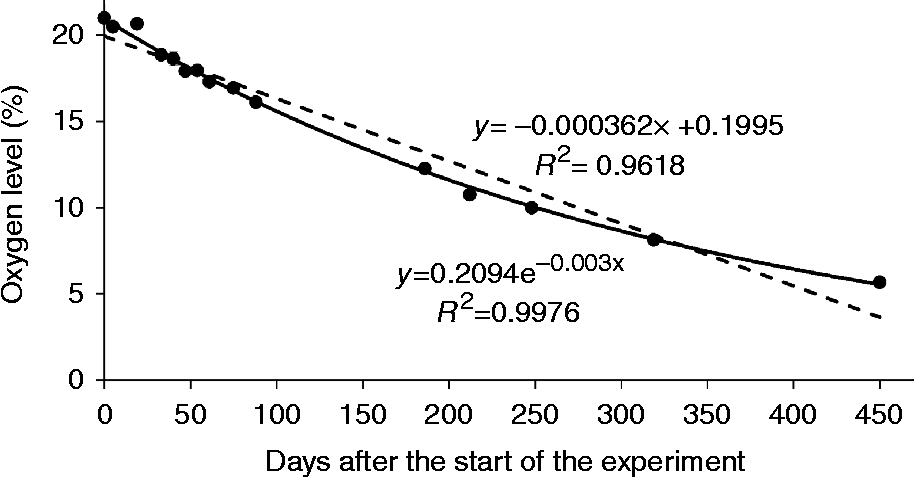
Fig. 4 Oxygen uptake by dry lettuce seeds equilibrated at 39% relative humidity (RH) and 20°C. The uptake was measured as the reduction in oxygen level with 10 g seeds in hermetically closed 47 ml jam jars. Standard errors of the measurements performed on two replicates were smaller than the symbols used in the graph. Trend lines are added, assuming either a linear (dashed) or an exponential (solid) relationship between oxygen level and experimental duration.
Effects of anoxia on seed viability
In the absence of an oxygen absorber and Drying Beads®, primed and pelleted celery and celeriac seeds stored at − 20°C produced 74 and 71% normal seedlings, respectively (Table 1). Similar hermetic storage in jam jars for 17 d, but at the relatively high temperature of 35°C, resulted in a considerable decline in the frequency of normal seedlings for celeriac, while the celery seeds failed to produce normal seedlings. The inclusion of only an oxygen absorber package in the containers failed to improve the viability of the celery seeds, whereas the frequency of normal seedlings for celeriac further declined to zero. Poor seed viability was also observed when only Drying Beads® were added to the containers with seeds. However, the addition of an oxygen absorber package as well as Drying Beads® to the seed containers maintained the frequency of normal seedlings, with values that were comparable to those observed for the control storage treatment at − 20°C.
Table 1 Effect of storage conditions on the frequency of normal seedlingsa
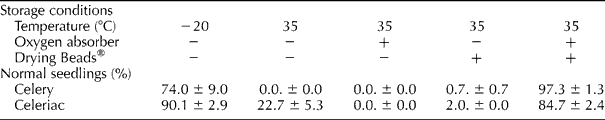
a Primed and pelleted celery and celeriac seeds were stored for 17 d in a closed jam jar with (+) or without ( − ) an oxygen absorber and with (+) or without ( − ) Drying Beads®. Three replicates of 50 seeds were used per treatment and standard error of the mean is reported.
Discussion
Seed deterioration
To optimize storage procedures to prolong seed longevity during ex situ conservation, it is important to consider the physiological and biochemical processes underlying the decline in seed viability. Loss of viability is caused by the gradual accumulation of molecular damage to, for example, cell membranes, proteins, DNA and mitochondria (Bewley et al., Reference Bewley, Bradford, Hilhorst and Nonogaki2013). Eventually, this damage results in the functional impairment of cells, tissues and organs. Under dry conditions, enzymatic repair cannot occur, resulting in the loss of viability through accumulating damage during dry storage. Related to the occurrence of reactive oxygen species (ROS), oxidation is the main form of molecular damage during the ageing of organisms (Harman, Reference Harman1956; Wilson and McDonald, Reference Wilson and McDonald1986; Hendry, Reference Hendry1993; Rajjou and Debeaujon, Reference Rajjou and Debeaujon2008). ROS that attack unsaturated fatty acids in membrane phospholipids may initiate lipid peroxidation, resulting in the alteration of membrane properties such as a decrease in fluidity, while membrane-bound proteins may also be disrupted. DNA oxidation by ROS may result in strand breaks and mutations (Cheah and Osborne, Reference Cheah and Osborne1978).
To counteract ROS-induced damage, cells possess an antioxidant defence system, which includes enzymes such as superoxide dismutase, catalase and glutathione peroxidase and molecular antioxidants to scavenge ROS. In dry seeds, enzyme activity is lacking or minimal, leaving molecular antioxidants as the main defence mechanism. The presence of antioxidants has probably been a driving force in plant evolution, as seeds contain relatively large amounts of various antioxidants, such as tocopherols (vitamin E), flavonoids and phenolic compounds. The importance of tocopherols for seed survival has been demonstrated by the strongly reduced seed longevity of a tocopherol-deficient Arabidopsis mutant (Sattler et al., Reference Sattler, Gilliland, Magallanes-Lundback, Pollard and DellaPenna2004). Seed deterioration is probably retarded in case of sufficient antioxidant capacity, but the rate of deterioration will increase once the antioxidant levels become (locally) critical or depleted. In dry seeds, antioxidants have limited mobility and tocopherols that are oxidized during storage cannot be regenerated or replaced by enzyme activity (Groot et al., Reference Groot, Surki, de Vos and Kodde2012).
Enzyme activity is only possible under moist conditions. In metabolically active plant tissues, endogenous ROS are produced during aerobic respiration by the mitochondrial electron transport chain, photo-production in chloroplasts and several enzymatic reactions (Giulivi et al., Reference Giulivi, Boveris and Cadenas1995; Asada, Reference Asada2006). These ROS include superoxide, peroxide and hydroxyl species and several nitrogenous species. It has been estimated that during soybean seed germination approximately 2% of the consumed molecular oxygen is converted to ROS (Puntarulo et al., Reference Puntarulo, Sánchez and Boveris1988). Most of these ROS may have a physiological function, but they may also harm the embryo. Consequently, antioxidant activity plays an important role in the regulation of ROS concentrations. For instance, maintenance of an appropriate ROS-scavenging system is essential to control ROS concentrations during imbibition and the onset of metabolic activity and germination. Therefore, extension of seed longevity depends on the ability to prevent (local) depletion of antioxidants due to interactions with ROS.
Because of the low levels of water activity, enzyme activity is not or hardly possible in seeds dried below 40% RH (Labuza, Reference Labuza1971). Consequently, oxygen consumption by aerobic respiration is also absent or very low. Therefore, oxygen uptake by dry seeds, such as that observed in the present study for lettuce (Fig. 4), is more likely related to the formation of superoxide or other ROS molecules and subsequent oxidation of macromolecules, such as lipids, phospholipids and DNA. Although ROS can also be generated by processes such as the autocatalytic lipid peroxidation pathway (Wilson and McDonald, Reference Wilson and McDonald1986; Walters, Reference Walters1998), interaction of molecular oxygen with, for example, metal ions or molecules in the seeds will be the main route for superoxide formation and oxygen uptake by dry seeds. Oxidation of organic matter is known to form a considerable threat to artefacts during the storage and display of museum collections (Arney and Jacobs, Reference Arney and Jacobs1979; Horie, Reference Horie1990). Oxidation during storage due to the presence of molecular oxygen has also been recognized as a problem by the food industry (Simon et al., Reference Simon, Labuza and Karel1971).
Because molecular oxygen is a source for ROS formation, its presence during seed storage can pose a serious threat to the survival of dry seeds. Therefore, it can be expected that, in addition to the antioxidant defence mechanism, species have evolved additional strategies for protection. For example, a large number of species are known to have developed seed-coats that exhibit low permeability for oxygen (Priestley et al., Reference Priestley, Werner and Leopold1985). The most exceptional longevity has been recorded for date palm seeds that were found to survive for about 2000 years after burial near the Dead Sea (Sallon et al., Reference Sallon, Solowey, Cohen, Korchinsky, Egli, Woodhatch, Simchoni and Kislev2008). The dry climate may have been a key factor, but the impermeable seed-coat and the high level of antioxidant activity generally observed in date palm seeds (Shams Ardekani et al., Reference Shams Ardekani, Khanavi, Hajimahmoodi, Jahangiri and Hadjiakhoondi2010) may have contributed to their long-term survival.
Anoxia and seed viability
Variable effects on viability have been reported by studies carried out using seeds stored under low oxygen concentrations or anoxic conditions, either through vacuum packaging or by creating a carbon dioxide- or nitrogen-enriched atmosphere. However, all these variable effects on viability can be ascribed to variation in the water activity of the seeds. Negative viability effects of anoxia are observed upon storage at relatively high seed moisture levels (Roberts and Ellis, Reference Roberts and Ellis1989). At high water activity levels, seeds are metabolically active and oxygen shortage will result in suffocation and anaerobic respiration, accompanied by the production of toxic acetaldehyde and ethanol. Studies carried out using dry seeds have reported either neutral or positive effects of anoxic storage on seed longevity (Gane, Reference Gane1948; Roberts and Abdalla, Reference Roberts and Abdalla1968; Shejbal, Reference Shejbal1979; Bennici et al., Reference Bennici, Bitonti, Floris, Gennai and Innocenti1984; Shrestha et al., Reference Shrestha, Shepherd and Turnbull1985; Barzali et al., Reference Barzali, Lohwasser, Niedzielski and Börner2005; González-Benito et al., Reference González-Benito, Pérez-García, Tejeda and Gomez-Campo2011; Schwember and Bradford, Reference Schwember and Bradford2011). The positive correlation between the oxygen level in the storage environment and the level of seed deterioration has also been shown in experiments in which an elevated oxygen concentration was created by hyperbaric storage (Ehrenberg et al., Reference Ehrenberg, Moutschen-Dahmen and Moutschen-Dahmen1957; Kronstad et al., Reference Kronstad, Nilan and Konzak1959; Moutschen-Dahmen et al., Reference Moutschen-Dahmen, Moutschen and Ehrenberg1959; Groot et al., Reference Groot, Surki, de Vos and Kodde2012).
The food industry is well aware of the negative effects of oxygen on seed quality as low oxygen conditions are recommended for the storage of consumptive seeds (Aurand and Woods, Reference Aurand and Woods1975; Maté et al., Reference Maté, Saltveit and Krochta1996; Jensen et al., Reference Jensen, Sorensen, Brockhoff and Bertelsen2003). Therefore, it is common practice in the food industry to pack seeds, such as those from sunflower, pumpkin or nuts, not only hermetically in oxygen-impermeable containers, but also under vacuum or under an anaerobic atmosphere to prevent the development of a ‘rancid’ taste caused by lipid oxidation. Seeds often contain polyunsaturated fatty acids, such as linoleic acid, which are rather susceptible to oxidation (Allen and Hamilton, Reference Allen and Hamilton1994). Hexanal produced during this process acts as an appropriate indicator of the degree of oxidation (Sanches-Silva et al., Reference Sanches-Silva, Rodríguez-Bernaldo de Quirós, López-Hernández and Paseiro-Losada2004). Upon hermetic packaging of pistachio nuts, hexanal production was shown to be highest for seeds stored under air, intermediate when vacuum-packed and lowest when an oxygen absorber was included (Leufven et al., Reference Leufven, Sedaghat and Habibi2008).
The experiment with primed and pelleted celery and celeriac seeds confirmed that the combination of a dry and anoxic environment is essential to prolong seed longevity (Table 1). The strong deteriorative effect observed upon adding only an oxygen absorber was most probably due to the moisturizer included in the package, causing a substantial increase in moisture levels. This is in line with the fact that deterioration was no longer observed when zeolite seed Drying Beads® were added. The primed celery and celeriac seeds appeared to be sensitive to severe drying as storage with only the Drying Beads® resulted in a severe decline in viability. These beads are known for their very strong capacity to absorb moisture from seeds (Hay et al., Reference Hay, Thavong, Taridno and Timple2012). The deteriorative effect of strong drying was not observed when oxygen was also removed. This is in agreement with studies showing that a reduction in seed longevity upon ultra-dry seed storage occurs in the presence of oxygen (Walters and Engels, Reference Walters and Engels1998) and that a quality decline upon hermetic storage of ultra-dry seeds is not observed in an oxygen-free or oxygen-limited environment (Steiner and Ruckenbauer, Reference Steiner and Ruckenbauer1995; Hong et al., Reference Hong, Ellis, Astley, Pinnegar, Groot and Kraak2005; González-Benito et al., Reference González-Benito, Pérez-García, Tejeda and Gomez-Campo2011).
Genebank management procedures
In addition to low temperature and low RH, hermetic closure of seed containers and anoxic conditions are recommended to prolong seed longevity during long-term storage. Our experiments confirm previous findings reported by Gómez-Campo (Reference Gómez-Campo2002) showing that Kilner jars and laboratory glass bottles provide adequate sealing against water vapour. We also observed no permeability for water vapour with the laminated foil bags and the jam jars closed with a metal lid and plastisol. The latter result is in contrast to the finding reported by Gómez-Campo (Reference Gómez-Campo2002), who observed leakage of jam jars after 3 years of testing. The discrepancy in results between the studies might be related to a more effective closure or the shorter experimental duration in the present study.
The Kilner jars, jam jars and laminated foil pouches are also impermeable to oxygen. This in contrast to plastic containers and glass bottles closed with either polypropylene or polybutylene terephthalate screw caps, which are permeable to oxygen to various degrees. When using plastic containers, or containers with plastic closure, the type of plastic should be carefully considered. Although all plastics have a certain degree of oxygen permeability, the level may strongly vary among the types of plastics. For example, the oxygen permeability of polypropylene is more than 50 times higher than that of polyethylene terephthalate (Massey, Reference Massey2003). In conclusion, laminated aluminium foil bags and glass jars with an impermeable lid are adequate for both oxygen-proof storage and prevention of the uptake of moisture from the environment.
Even when oxygen-proof containers are used, the amount of oxygen included should be limited or reduced upon storage (Fig. 1). Several methods can be employed to create anoxic conditions or to lower oxygen concentrations:
-
(1) ‘Modified atmosphere packaging’: This method is frequently used by the food industry for the packaging of seeds through gas-flushing or compensated vacuum packaging (Church and Parsons, Reference Church and Parsons1995). During gas-flushing, the package is flushed with nitrogen or carbon dioxide, while in compensated vacuum packaging most of the air is removed before inclusion of the desired gas. The advantage of using nitrogen gas is the avoidance of any potential acidification, as might be the case for carbon dioxide depending on the moisture level.
-
(2) Vacuum packaging in flexible laminated aluminium foil bags: This method is already employed by several genebanks, including the CGN. Apart from the reduced volume, an additional advantage of vacuum packaging is that sporadic puncturing of the packaging material can easily be noticed by disruption of the vacuum.
-
(3) Inclusion of oxygen absorbers: Oxygen levels can also be reduced by inclusion of an oxygen absorber package. The active ingredient in oxygen absorbers is iron powder. Oxygen absorbers are, for instance, used by the food industry for the packaging of bakery products. As has been shown in the present study, the use of additional moisturizers should be avoided when using this method to conserve dry seeds. Oxygen absorbers without moisturizers are used, for example, for the packaging of pharmaceutical products and electronic equipment.
-
(4) Oxygen consumption by seeds during storage: After hermetic storage, oxygen levels can be lowered through consumption by the seeds (Fig. 4). However, compared with packaging under anoxic conditions, oxygen consumption by seeds will result in a reduction of the total antioxidant capacity. In contrast to moist seeds, dry seeds are unable to regenerate antioxidants during storage (Groot et al., Reference Groot, Surki, de Vos and Kodde2012). Once antioxidant capacity is locally depleted, seed damage is likely to be initiated. Further experimental studies are needed to quantify the rate of decline in antioxidant capacity during seed storage in relation to moisture level, temperature and oxygen pressure.
Genebank logistics usually requires temporary seed storage between harvest and long-term storage, e.g. for quality assessment, which may last for a few weeks to several months. Although usually performed under relatively low temperature and humidity conditions, this temporary seed storage rarely takes place under hermetic and anoxic conditions. In ‘open storage’, the seeds are exposed to an unlimited supply of oxygen and the potential longevity of the seeds will be reduced through a decline in their antioxidant capacity (Groot et al., Reference Groot, Surki, de Vos and Kodde2012). Most seeds have large quantities of antioxidants, which will mask an initial decline in potential seed longevity. Also, limited damage during storage is often only evident from a delay in radicle protrusion, while total germination is not or hardly affected. However, studies carried out using laboratory bench-stored cabbage seeds have shown a decline in mitochondrial quality already after 3 months of storage, without any visible effects on seed quality in a germination assay (Kodde et al., Reference Kodde, Buckley, de Groot, Retiere, Viquez Zamora and Groot2011). Seeds have the capacity to repair damage after sowing. Only after prolonged storage when damage surpasses certain thresholds, seeds fail to produce healthy seedlings. This limited seed deterioration can be acceptable for most commercial seed lots as they are usually stored for only a few years. However, any reduction in viability compromises the general aim of genebanks to maximize the longevity of seed samples. Therefore, anoxic storage soon after drying is recommended for genebanks.
The use of anoxia or reduced oxygen levels for temporary seed storage may also be relevant for primed seeds with a short shelf life as for those from celery and celeriac (Table 1) and for commercial seed lots in the tropics, where cool storage is expensive and unreliable in case of power failures. Drying and cooling soon after harvest will improve seed longevity considerably. Shortening the period during which seeds are exposed to oxygen as much as possible will further extend seed longevity.
Conclusions
A growing body of evidence has emerged about the deleterious effects of oxygen on seed longevity during the storage of dry seeds. This finding has so far received little attention in the optimization of genebank management procedures. To prolong the longevity of ex situ conserved seed samples, we recommend that seeds be stored under dry and cool conditions with no, or at most limited, availability of oxygen as soon as possible after harvesting and drying. For long-term storage, the seed storage containers should be impermeable to both moisture and oxygen, while the level of included oxygen should be reduced through vacuum packaging, nitrogen flushing or inclusion of dry oxygen absorbers.
Acknowledgements
This work was carried out in the framework of the Fundamental Research Program on Sustainable Agriculture (KB-12-005.03-004 and BO-12.03-017) funded by the Dutch Ministry of Economic Affairs. The authors thank the seed company Rijk Zwaan (de Lier, the Netherlands) for providing the seed samples, Mitsubishi Gas Chemical Company, Inc. (Tokyo, Japan) for providing the Ageless® oxygen absorbers, Rhino Research (Phichit, Thailand) for providing the zeolite seed Drying Beads® and the Millennium Seed Bank Project (Kew, UK) for providing their storage glass vessels.







