A promising approach to clarifying linkages between biological systems/processes and mental health problems (cf. Kozak & Cuthbert, Reference Kozak and Cuthbert2016) is to focus on clinically relevant traits quantified in neurobehavioral (NB) terms. Traits quantified in this manner, termed NB traits (Depue & Iacono, Reference Depue and Iacono1989; Patrick, Venables et al., Reference Patrick, Venables, Yancey, Hicks, Nelson and Kramer2013), are conceptualized as individual difference characteristics that evidence direct, empirical links to both neural-response and clinical-symptom variables to provide an interface between these two realms (Perkins, Latzman, & Patrick, Reference Perkins, Latzman and Patrick2019). There is wide agreement that full understanding of psychological phenomena depends upon comprehensive assessment across multiple units of analysis, spanning from genomic variation through observable behavior and self-reported experience (Cuthbert & Insel, 2013). A construct-based multimodal measurement approach underscores the utility of NB-trait constructs as anchors for integrating measures from brain response, task performance, and self-report modalities (Patrick, Iacono, & Venables, Reference Patrick, Iacono and Venables2019). To date, this approach has been used to operationalize the NB traits of inhibitory control (Patrick et al., Reference Patrick, Venables, Yancey, Hicks, Nelson and Kramer2013; Venables et al., Reference Venables, Foell, Yancey, Kane, Engle and Patrick2018), threat sensitivity (Yancey, Venables, & Patrick, Reference Yancey, Venables and Patrick2016), and reward sensitivity (Bowyer et al., Reference Bowyer, Joyner, Yancey, Venables, Hajcak and Patrick2019), which evidence relations with externalizing problems, fear disorders, and depressive disorders, respectively. The current study was undertaken as a step toward a multimodal measurement model for the construct of affiliative capacity (AFF). Our aim was to operationalize this construct using trait scale and neural-response variables and demonstrate associations of this combined measure with clinical-symptom and neurophysiological criterion variables.
The NB trait of AFF is defined here as variation in the ability and desire to establish social–emotional bonds with others (e.g., Waller & Wagner, 2019). The use of the term “capacity” suggests the availability of affiliative resources – regardless of an individual’s tendency to seek out interpersonal relationships – and involve foundational neurobiological processes (i.e., mechanistic precursors) before they are influenced by other major systems of functioning. Low levels of this trait can manifest as social detachment or disinterest, callous disregard of others, deficient empathy, and blunted social responsiveness and emotional expressivity – and are evident in a variety of clinical conditions, including antagonistic externalizing (e.g., conduct disorder [CD], antisocial personality, narcissistic personality, and psychopathy; Frick, Ray, Thornton, & Kahn, Reference Frick, Ray, Thornton and Kahn2014; Lynam, Miller, & Derefinko, Reference Lynam, Miller and Derefinko2018; Paulhus & Williams, Reference Paulhus and Williams2002), internalizing (e.g., major depressive disorder; Cusi, MacQueen, Spreng, & McKinnon, Reference Cusi, MacQueen, Spreng and McKinnon2011; Schrepferman, Eby, Snyder, & Stropes, Reference Schrepferman, Eby, Snyder and Stropes2006), psychotic (e.g., schizophrenia; Walker, Kessler, Bollini, & Hochman, Reference Walker, Kesstler, Bollini and Hochman2004), and neurodevelopmental (e.g., autism spectrum; Dawson & Bernier, Reference Dawson and Bernier2013) disorders. This evidence for transdiagnostic relevance, along with links to biological conceptualizations of social behavior, points to AFF as a useful construct for linking dimensions of psychopathology with neurobiological systems and processes.
Characterizing psychopathology in terms of mechanisms and dimensions
A major emphasis in contemporary theories of psychopathology, given pervasive evidence for systematic overlap (comorbidity) among different mental disorders, is on transdiagnostic mechanisms – i.e., deviations in cognitive, emotional, and/or behavioral functioning contributing to various disorders (Barlow, Allen, & Choate, Reference Barlow, Allen and Choate2016; Ehring & Watkins, Reference Ehring and Watkins2008; Harvey & Watkins, Reference Harvey and Watkins2004; Insel & Cuthbert, Reference Insel and Cuthbert2009; Kring & Sloan, Reference Kring and Sloan2009; Mansell, Harvey, Watkins, & Shafran, Reference Mansell, Harvey, Watkins and Shafran2009; Nolen-Hoeksema & Watkins, Reference Nolen-Hoeksema and Watkins2011). One salient example of this is the National Institute of Mental Health’s Research Domain Criteria (RDoC) framework (Kozak & Cuthbert, Reference Kozak and Cuthbert2016), which calls for investigation of transdiagnostic mental health problems in terms of basic biobehavioral processes, quantified using measures of various types. An accompanying trend in the field involves the use of quantitative modeling to characterize and quantify clinical problems in terms of lower and higher order dimensions (e.g., the Hierarchical Taxonomy of Psychopathology [HiTOP]; Kotov et al., Reference Kotov, Krueger, Watson, Achenbach, Althoff, Bagby and Zimmerman2017; Krueger et al., Reference Krueger, Kotov, Watson, Forbes, Eaton, Ruggero and Zimmermann2018), as an alternative to traditional categorical systems for psychopathology (e.g., Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association, 2013). These two complementary movements in the field create exciting opportunities for moving toward an integrative science of psychopathology. To progress effectively in this direction, strategic approaches are needed for integrating measures from different response modalities (e.g., self-report, task performance, central, and peripheral physiology) to index variations in biobehavioral functioning that relate to narrower versus broader dimensions of symptomatology.
We next describe a novel research paradigm – the psychoneurometric approach (Patrick, Durbin, & Moser, Reference Patrick, Durbin and Moser2012; Patrick, Iacono, & Venables, Reference Patrick, Iacono and Venables2019) – for integrating variables of different types into multimodal measures of clinically relevant NB traits. Following this, we discuss the NB trait of AFF (social warmth vs. callousness [CA]) and consider brain response measures that operate as indicators of this trait dimension.
Psychoneurometric research approach
The biological, psychological, and socioemotional factors that influence psychopathology are interdependent, and research limited to a single measurement modality hinders progress in fully understanding psychological phenomena (Anderson, Reference Anderson1998). In psychological science, constructs are typically operationalized using self-report questionnaires, which then serve as criteria for identifying construct-relevant indicators from other modalities of measurement (e.g., neural response). However, correlations between measures from different modalities – such as self-report and brain response – are typically only modest in magnitude, due to the presence of modality-specific variation in scores (i.e., method variance; Campbell & Fiske, Reference Campbell and Fiske1959). In view of this, specialized approaches are needed to effectively characterize a construct’s network of associations with variables spanning different levels (Cacioppo & Berntson, Reference Cacioppo and Berntson1992) or units of analysis (Kozak & Cuthbert, Reference Kozak and Cuthbert2016).
The psychoneurometric research strategy (Patrick et al., Reference Patrick, Durbin and Moser2012, Reference Patrick, Venables, Yancey, Hicks, Nelson and Kramer2013, Reference Patrick, Iacono and Venables2019) provides a means to address the issue of method variance. It focuses on NB traits – dispositional constructs with biological and behavioral as well as psychological referents – as anchors for integrating indicators from different assessment domains into multimodal trait measures (e.g., threat sensitivity, Yancey et al., Reference Yancey, Venables and Patrick2016; inhibitory control, Venables et al., Reference Venables, Foell, Yancey, Kane, Engle and Patrick2018). The approach to operationalizing traits of these types proceeds in a series of steps that lead to progressive refinement of the initial conceptualization of the trait (see Patrick et al., Reference Patrick, Iacono and Venables2019). Effort is first devoted to identifying reliable indicators from physiological and/or task-behavioral modalities of the trait as assessed (provisionally) through self-report. Following this, analyses can be undertaken to evaluate the covariance structure of such indicators, in order to clarify their functional meaning (e.g., through consideration of distinct processing demands of the laboratory tasks they derive from) and refine multimodal quantification of the trait. This is followed by efforts to (a) update conceptualization of the trait to incorporate insights gained from structural analyses of non-report (physiological, behavioral) indicators, (b) modify the self-report scale measure of the trait to reflect this updated conceptualization, and (c) use knowledge of the functional meaning of the non-report indicators to identify other such indicators of the trait. This process continues iteratively until an optimal set of indicators exists for operationalizing the trait in a precise and reliable manner (for additional details, and a schematic depiction of steps, see Patrick et al., Reference Patrick, Iacono and Venables2019).
The psychoneurometric approach has been successfully used to operationalize NB traits of inhibitory control, threat sensitivity, and reward sensitivity, which have proven useful for interfacing clinical outcomes with neurophysiological and behavioral measures. Venables et al. (Reference Venables, Foell, Yancey, Kane, Engle and Patrick2018) reported on the development of such a model for the NB construct of inhibitory control utilizing indicators from measurement modalities of self-report, neurophysiology, and behavioral task performance. A structural model of these indicators accounted for patterns of covariance among them and further demonstrated clinical relevance to externalizing problems, specifically. In other work, Yancey et al. (Reference Yancey, Venables and Patrick2016) showed that threat sensitivity, quantified jointly using physiological and self-report indicators, exhibited similar-level correlations with fear-disorder symptom and physiological criterion measures. More recently, Bowyer et al. (Reference Bowyer, Joyner, Yancey, Venables, Hajcak and Patrick2019) combined scores on a trait-dysphoria measure with an electrocortical measure of reward–feedback response to index of reward sensitivity and demonstrated selective relations for this index with depressive as compared to phobic fear symptomatology.
Conceptualizing and assessing AFF in NB-trait terms
As noted earlier, AFF – defined as variation in the desire for and ability to establish social–emotional bonds with others – has transdiagnostic significance given its relevance to different clinical conditions involving deficient empathy and detachment (i.e., broad externalizing, distress-based internalizing, autism spectrum, and schizophrenia). In addition, AFF has clear biological and behavioral referents: situated within the Social Processes domain of the RDoC framework (Kozak & Cuthbert, Reference Kozak and Cuthbert2016), affiliation depends on various neurocognitive processes, including accurate detection and interpretation of social cues, as well as social learning and memory processes supporting the formation and maintenance of interpersonal relationships. Further, as discussed below, it shows replicable neural correlates when defined as dispositional CA versus empathic concern. As such, AFF appears amenable to multimodal quantification.
CA, the most commonly studied dispositional expression of low AFF, has been increasingly recognized as relevant to social dysfunction across multiple clinical conditions. This trait is characterized by a disinterest in or atypical motivation for the formation and maintenance of social relationships, a lack of empathy for others’ distress, and uncaring and unemotional tendencies reflecting insensitivity to the needs and feelings of others (Barry et al., Reference Barry, Frick, DeShazo, McCoy, Ellis and Loney2000; Berg et al., Reference Berg, Lilienfeld, Reddy, Latzman, Roose, Craighead and Raison2013; Frick, Barry, & Bodin, Reference Frick, Barry, Bodin and Gacono2000; Frick, Cornell, Barry, Bodin, & Dane, Reference Frick, Cornell, Barry, Bodin and Dane2003; Hyde, Burt, Shaw, Donnellan, & Forbes, Reference Hyde, Burt, Shaw, Donnellan and Forbes2015; Patrick, Fowles, & Krueger, Reference Patrick, Fowles and Krueger2009). Much of the early work on CA focused on school-age and early adolescent samples (Frick, Cornell, et al., Reference Frick, Cornell, Barry, Bodin and Dane2003; Frick, O’Brien, Wootton, & McBurnett, Reference Frick, O’Brien, Wootton and McBurnett1994), with more recent studies extending this work to earlier childhood (children aged 4 to 8; Hawes & Dadds, Reference Hawes and Dadds2007) and later adolescence (aged 13 to 18; Essau, Sasagawa, & Frick, Reference Essau, Sasagawa and Frick2006). Evidence that CA is “trait-like” comes from findings that CA is highly heritable (Viding, Blair, Moffitt, & Plomin, Reference Viding, Blair, Moffitt and Plomin2005; Viding, Jones, Frick, Moffitt, & Plomin, Reference Viding, Jones, Frick, Moffitt and Plomin2008), emerges early in life (Hawes & Dadds, Reference Hawes and Dadds2007), and remains relatively stable across development (i.e., ages 4 to 13; Dadds, Fraser, Frost, & Hawes, Reference Dadds, Fraser, Frost and Hawes2005; Frick, Kimonis, Dandreaux, & Farell, Reference Frick, Kimonis, Dandreaux and Farell2003; Muñoz & Frick, Reference Muñoz and Frick2007; Obradović, Pardini, Long, & Loeber, Reference Obradović, Pardini, Long and Loeber2007).
Work with child and adolescent samples has demonstrated salient associations of CA with personality characteristics and clinical outcomes (Burke, Loeber, & Lahey, Reference Burke, Loeber and Lahey2007). Youth with callous traits evidence distinct emotional, cognitive, and temperamental characteristics that distinguish them from other antisocial youth (Frick & White, Reference Frick and White2008). Further, CA helps characterize a subgroup of youth with a specific vulnerability to conduct problems and antisocial personality disorder (McMahon, Witkiewitz, Kotler, & The Conduct Problems Prevention Research Group, Reference McMahon, Witkiewitz and Kotler2010; Frick & White, Reference Frick and White2008; Rowe et al., Reference Rowe, Maughan, Moran, Ford, Briskman and Goodman2010). Drawing on these lines of evidence, the recent fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; American Psychiatric Association, 2013) includes a CA specifier for CD, termed “limited prosocial emotions” (McMahon et al., Reference McMahon, Witkiewitz and Kotler2010; Frick & Moffitt, Reference Frick and Moffitt2010).
Beyond antisocial behavior, dispositional CA has been linked to internalizing conditions marked by pervasive distress (e.g., Essau et al., Reference Essau, Sasagawa and Frick2006; Barker & Salekin, Reference Barker and Salekin2012) and internalizing-related temperament traits related to such conditions (e.g., Latzman, Lilienfeld, Latzman, & Clark, Reference Latzman, Lilienfeld, Latzman and Clark2013; Berg et al., Reference Berg, Lilienfeld, Reddy, Latzman, Roose, Craighead and Raison2013). The positive relations found between CA and both internalizing and externalizing psychopathology involving interpersonal problems suggest that callous traits relate to social withdrawal and isolation, lack of attachment, and low mood (Gao & Zhang, Reference Gao and Zhang2016), consistent with the RDoC conceptualization of low affiliation more broadly.
Of importance to the current work, evidence from behavioral, neuroimaging, and neurophysiological modalities supports the notion that CA, as a manifestation of AFF−, involves a deficit in social processing, specifically in the ability to recognize and respond to others’ distress (Shirtcliff et al., Reference Shirtcliff, Vitacco, Graf, Gostisha, Merz and Zahn‐Waxler2009; Marsh et al., Reference Marsh and Blair2008; Marsh, Reference Marsh2016; Viding et al., Reference Viding, Sebastian, Dadds, Lockwood, Cecil, De Brito and McCrory2012; Brislin & Patrick, Reference Brislin and Patrick2019). For example, children, adolescents, and adults high in trait CA show reduced accuracy in categorizing emotional faces as fearful or sad (Marsh & Blair, Reference Marsh and Blair2008; White et al., 2015) and amygdala hypoactivity to fearful faces, in particular (Marsh & Blair Reference Marsh and Blair2008; Marsh, Reference Marsh2016; Viding et al., Reference Viding, Sebastian, Dadds, Lockwood, Cecil, De Brito and McCrory2012). More recently, in adult samples, self-reported CA has been linked to reductions in brain- event-related potential (ERP) reactivity to fearful faces (Brislin & Patrick, Reference Brislin and Patrick2019; Brislin et al., Reference Brislin, Yancey, Perkins, Palumbo, Drislane, Salekin and Patrick2018). Specifically, individuals high in CA show blunted amplitude of ERP components thought to reflect face detection/categorization and emotional encoding processes – namely, N170 and P2, respectively (Shannon, Patrick, Venables, & He, Reference Shannon, Patrick, Venables and He2013).
Taken together, reliable and valid self-report CA scales, already established in the literature (e.g., Patrick, Kramer, Krueger, & Markon, Reference Patrick, Kramer, Krueger and Markon2013), have served as an anchor for research on the neural correlates of AFF (i.e., biomarkers of facial emotional processing, N170, and P2; Brislin & Patrick, Reference Brislin and Patrick2019; Brislin et al., Reference Brislin, Yancey, Perkins, Palumbo, Drislane, Salekin and Patrick2018). These findings suggest that social–emotional processing deficits associated with dispositional CA can be indexed psychometrically to facilitate the shift of the AFF− vector into the cross-modal space that is more representative of the underlying construct and may not be accessible solely through the use of the self-report or behavioral measures.
The present study
The current work sought to demonstrate that AFF− can be effectively operationalized using neurophysiological along with self-report indicators, to lay the groundwork for a multimodal model of this trait construct that can help to clarify relations among dispositional affiliative tendency, social processes in the brain, and transdiagnostic dimensions of clinical problems. Building on prior research findings (Brislin et al., Reference Brislin, Yancey, Perkins, Palumbo, Drislane, Salekin and Patrick2018; Patrick, Venables et al., Reference Patrick, Venables, Yancey, Hicks, Nelson and Kramer2013; Yancey et al., Reference Yancey, Venables and Patrick2016), the current study used data from a sample of adult twins to (1) integrate self-report and neural indicators to create a psychoneurometric index of low AFF (AFF−) and (2) evaluate the reliability of this psychoneurometric index and its effectiveness for predicting specific clinical conditions assessed via interview (i.e., antagonistic personality disorders and internalizing disorders), broad dimensions of psychopathology (Kotov et al., Reference Kotov, Krueger, Watson, Achenbach, Althoff, Bagby and Zimmerman2017), and physiological criterion measures (i.e., indices of emotional processing). Considering the shared social disaffiliation characteristic of externalizing and distress dimensions of psychopathology (Gao & Zhang, Reference Gao and Zhang2016), it was hypothesized that AFF− would be associated with these two broad dimensions, and the respective diagnostic symptom counts, to a moderate and weak degree, respectively. Fear symptoms were not expected to be associated with AFF−.
1. Method
1.1. Participants
The base sample consisted of 508 same-sex adult twins (257 females; 44.2% monozygotic, 38.1% dizygotic, 17.7% individuals without a twin included in the study) recruited from the greater Minneapolis-St. Paul metro area. Half of these participants were selected from a larger screening sample (N = 2511) based on their scores on a fear/fearlessness inventory. Specifically, one-third were chosen to be high (i.e., highest 18% of the screening sample), one-third low (i.e., lowest 18%), and the remaining third in the intermediate range (i.e., 19th to 82nd percentile). The co-twins of these pre-selected individuals comprised the other half of the sample. Participants were also evaluated for and determined to be free from visual or hearing impairments.
From among the base sample of 508, 32 subjects were missing in the dispositional self-report measure of AFF− and 267 others were missing data for one of the two physiological indicators described below. For these participants, full information maximum likelihood estimation (as implemented in Mplus 6; Muthén & Muthén, Reference Muthén and Muthén2011) was used to generate imputed score values for the missing indicator, as supported by various simulation studies (Lee & Huber, Reference Lee and Huber2011; Little, Jorgensen, Lang, & Moore, Reference Little, Jorgensen, Lang and Moore2014; Schafer & Graham, Reference Schafer and Graham2002; Dong & Peng, Reference Dong and Peng2013). One participant who was missing more than one indicator was excluded from the analyses, resulting in an N value of 507 (50.5% female, M age = 29.5 years, SD = 4.8) for the reported analyses. The racial composition of this analysis sample was as follows: 96.4% White/Caucasian, 0.8% Black/African, 2.8% Other, with 0.4% of the sample identifying as Hispanic.
Study procedures were approved by the University of Minnesota’s Institutional Review Board, and the written informed consent was obtained from each participant prior to testing. Participants were paid $100 for the completion of testing, which was conducted within a single session and included a diagnostic interview, a laboratory-based physiological assessment, and administration of questionnaire measures.
1.2. Dispositional and diagnostic measures
1.2.1. Externalizing spectrum inventory: CA
Callous tendencies were assessed using items from the Externalizing Spectrum Inventory (ESI; Krueger, Markon, Patrick, Benning, & Kramer, Reference Krueger, Markon, Patrick, Benning and Kramer2007), a questionnaire designed to index differential expressions of externalizing tendencies. A 100-item abbreviated version (ESI-100; see Patrick et al., Reference Patrick, Venables, Yancey, Hicks, Nelson and Kramer2013) was administered to the current study sample. A subset of 25 items – consisting of items from the following ESI scales, which loaded .3 or higher on the callous-aggression factor of the ESI (Krueger et al., Reference Krueger, Markon, Patrick, Benning and Kramer2007) – was used to index CA (CA): Empathy (-); Relational, Destructive, and Physical Aggression; Excitement Seeking; Rebelliousness; and Honesty (-). CA scale scores were computed as the mean response across these 25 items, each coded 0 to 3, after reverse-coding negatively worded items – so that higher scores reflected greater callous-aggressive tendencies. Internal consistency reliability (Cronbach’s alpha) for this scale in the current sample was .79.
1.2.2. Structured clinical interview for DSM-IV Axis I and II Disorders
All participants were assessed for the full range of lifetime DSM-IV Axis I anxiety, mood, and substance disorders, as well as Axis II personality disorders, using the Structured Clinical Interview for DSM-IV Axis I Clinical Disorders and the counterpart interview for Axis II Personality Disorders (SCID-I and SCID-II; First, Spitzer, Gibbon, & Williams, Reference First, Spitzer, Gibbon and Williams1997; First, Gibbon, Spitzer, Benjamin, & Williams, Reference First, Gibbon, Spitzer, Benjamin and Williams1997). Each participant was interviewed by a PhD-level clinical psychologist or advanced clinical psychology graduate student trained in the administration and scoring of the SCID diagnostic interview. Interviewers had no knowledge of other assessment data collected from interviewees in earlier sessions. The SCID interview contains questions assessing individual symptom criteria for each disorder, with each rated as present, subthreshold, or absent by the interviewer. Symptom ratings were assigned through a consensus-diagnosis process (cf. Iacono, Carlson, Taylor, Elkins, & Mcgue, Reference Iacono, Carlson, Taylor, Elkins and Mcgue1999) entailing meetings of the interviewers with the project principal investigator (CJP) and a licensed clinical psychologist who provided consultation on ratings and diagnostic decisions.
Data from the SCID-I and SCID-II can be used to assign categorical diagnoses (present vs. absent) for each of the disorders, or to compute continuous symptom counts for each (i.e., the maximum number of diagnostic criteria met at any time in the individual’s life). For purposes of examining relations with affiliative trait scale scores and physiological response measures, the present study used diagnostic symptom counts as the main unit of analysis for each of the following clinical conditions from the Antagonistic Externalizing spectrum of HiTOP (Kotov et al., Reference Kotov, Krueger, Watson, Achenbach, Althoff, Bagby and Zimmerman2017): adult antisocial behavior (AAB), CD, narcissistic personality disorder (NPD), borderline personality disorder (BPD), and histrionic personality disorder (HPD). This spectrum was chosen as it is considered to encompass traits relating to dispositional low AFF (i.e., lack of empathy and social motivation, aggression, deceitfulness, manipulativeness, and egocentricity). Symptom count averages (standardized on a 0–1 scale) for these five disorders were as follows: AAB, .17; CD, .07; NPD, .13; BPD, .10; and HPD, .08.
Additionally, as noted above, it was hypothesized that low AFF would be associated to some extent with distress-related internalizing disorders (Clark & Watson, Reference Clark and Watson2006; Kotov et al., Reference Kotov, Krueger, Watson, Achenbach, Althoff, Bagby and Zimmerman2017), which involve social withdrawal and anhedonia. To examine this, symptom counts were computed for the following distress disorders: major depressive disorder (worst depressive episode), generalized anxiety disorder (GAD), and post-traumatic stress disorder (PTSD). For purposes of evaluating discriminant validity with respect to criterion measures not predicted to relate to low AFF, symptom counts were also computed for fear disorders (social phobia, specific phobia, panic disorder, and agoraphobia; see (Clark & Watson, Reference Clark and Watson2006; Kotov et al., Reference Kotov, Krueger, Watson, Achenbach, Althoff, Bagby and Zimmerman2017). Symptom count averages (standardized on a 0–1 scale) for these disorders were as follows: major depressive episode (MDE), .21; GAD, .06; PTSD, .06; social phobia, .22; specific phobia, .12; panic, .05; and agoraphobia, .02. Symptom composites were also computed for distress and fear pathology dimensions (or “subfactors”; Kotov et al., Reference Kotov, Krueger, Watson, Achenbach, Althoff, Bagby and Zimmerman2017) – in each case consisting of the average of symptom scores across individual disorders within each category. Due to missing data for certain diagnostic criterion measures, the sample size for validity coefficients ranges from 418 to 507 for interview-based clinical problem measures.
1.3. Procedure and experimental task paradigms
Data for the current study were collected as part of a test protocol that included physiological measurement tasks along with questionnaire and interview assessments. Seated in a padded recliner, participants completed a set of questionnaires (including the ESI-100), while an electroencephalographic (EEG) cap and skin-surface electrodes were attached to record brain response and peripheral physiological response (facial electromyography and heart rate) data, respectively. During testing, participants viewed the task stimuli on a 53.3-cm computer monitor, situated 1 m away at eye level. Stimuli were presented using a PC computer running E-Prime software (Psychology Software Tools, Inc.), and physiological data were collected using a second PC running Scan 4 software (Neuroscan, Inc., Herndon, VA, USA). Task order was consistent across participants, though trial order within each task was counterbalanced. The Binocular Rivalry task (BR; described below) was included partway through data collection, and thus, a subset of participants does not have data for this task. The current physiological indicators (i.e., N170 and P2) have been implicated as biomarkers of facial emotional processing, which plays a critical underlying role in social affiliation (e.g., Blair, Leibenluft, & Pine, Reference Blair, Leibenluft and Pine2014; Brislin & Patrick, Reference Brislin and Patrick2019; Brislin et al., Reference Brislin, Yancey, Perkins, Palumbo, Drislane, Salekin and Patrick2018). To reduce the potentially confounding effect of task-specific variance in the development of the multimodal measure, the ERPs were derived from two separate experimental tasks.
1.3.1. Emotional Stroop task (E-Stroop)
One of the physiological measurement tasks was an emotional conflict paradigm in which fearful and happy facial expressions were presented with the words “happy” or “fear” superimposed across them (cf. Etkin, Egner, Peraza, Kandel, & Hirsch, Reference Etkin, Egner, Peraza, Kandel and Hirsch2006). The task consisted of 148 presentations of happy or fearful facial expression photographs selected from the Ekman & Friesen (Reference Ekman and Friesen1976) stimulus set. Stimuli were presented for 1000 ms each, with a varying interstimulus interval of 3000–5000 ms (M = 4000 ms) during which a central fixation cross was shown. Stimuli were counterbalanced such that there were no consecutive presentations of the same face, either with same or with differing word distracters. Participants were asked to identify the emotional expression of each face, while disregarding the co-occurring word, which was either congruent or incongruent with the expression. To test our hypothesis that callous dispositional traits would be associated with reduced ERP responses to fearful expressions specifically, current analyses focused only on the data for fear-face trials (i.e., N170 and late positive potential [LPP] responses to fearful face stimuli; see “Data Processing and Variable Quantification”).
1.3.2. Binocular Rivalry task
In this task, participants viewed face stimuli under two conditions, blocked into separate trials, while wearing stereoscopic glasses: standard viewing trials, in which the same face image was presented to both eyes, and “suppressed” viewing trials, in which a face image was presented to one eye and masked by presentation of a 20-Hz Mondrian “noise” pattern to the other eye (see Shannon et al., Reference Shannon, Patrick, Venables and He2013, for further details). The face stimuli consisted of fearful and neutral expressions, posed by different actors, from the standardized NimStim face set and were presented for 500 ms each. The P2 brain response to fear-face stimuli, an early positive-going component of the ERP elicited by face stimuli, was extracted for the standard viewing trials for faces of this type (Jiang et al., Reference Jiang, Shannon, Vizueta, Bernat, Patrick and He2009).
1.3.3. Affective picture-viewing task
This task involved passively viewing 90 color-photographic stimuli (30 neutral, 30 aversive, 30 unpleasant) from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, Reference Lang, Bradley and Cuthbert1997). Each picture stimulus was counterbalanced and presented for 6 s, followed by an interval of 12 s preceding the next picture presentation, during which a fixation cross was displayed. Neutral pictures included household objects, buildings, and neutral faces (10 of each). Aversive scenes included 20 threat pictures (aimed guns and attacking animals) and 10 mutilation pictures (injured bodies, limbs, and faces). Pleasant pictures included erotic, nurturing (babies and small animals), and adventure scenes (10 of each).
During 81 of the 90 picture stimuli, noise probes (50 ms, 105 dB, 10 μs rise time) were presented at 3, 4, or 5 s into the 6 s picture presentation interval, to elicit startle-blink responses (see Yancey, Vaidyanathan, & Patrick, Reference Yancey, Vaidyanathan and Patrick2015, for startle-blink findings from this sample). Within and between orders, picture stimuli and noise probes were counterbalanced such that all picture valence categories (pleasant, neutral, and aversive) were represented equally across orders at each serial position, with the following constraints: no more than two pictures of the same valence occurred consecutively within any stimulus order; pictures of the same content category never appeared consecutively or across orders, and pictures were rotated so as to serve in both probed and unprobed conditions. The physiological criterion measure derived from this task (as described below) was differential LPP response to aversive as compared to neutral picture stimuli.
1.3.4. Visual oddball task
This task included counterbalanced stimuli of three types, presented for 100 ms each and separated by 4–5 s intervals: frequent non-targets (simple ovals; 70% of 240 total trials), infrequent targets (schematic heads; 15%), and infrequent novels (affective and neutral IAPS pictures; 15%); a detailed description of the task is reported in Yancey, Venables, Hicks, and Patrick (Reference Yancey, Venables, Hicks and Patrick2013). Participants responded with a left or right button-press on target (head) trials to indicate the location of an “ear,” on either the left or right side of the head. As described further below, the target P3 was extracted from this task as a measure of discriminant validity.
1.4. Data acquisition
EEG and electromyography activities were recorded from 54 scalp sites using Neuroscan Synamps 2 amplifiers and sintered Ag-AgCl EEG electrodes, positioned within a head-cap in accordance with the 10–20 system (Jasper, Reference Jasper1958). Separate electrodes were placed above and below the left eye to monitor vertical electrooculogram activity, and adjacent to the outer canthi of the left and right eyes to monitor horizontal electrooculogram activity to subsequently correct for eye movement. All electrode impedances were kept below 10 kΩ. The raw EEG signal was continuously recorded at a rate of 1000 Hz with an analog bandpass filter of 0.05 to 200 Hz.
1.5. Data processing and variable quantification
Data were referenced to electrode site Cz during data collection and arithmetically re-referenced offline to the average of left and right mastoid electrodes for subsequent processing and analysis. Data epochs from −1000 to 2000 ms were extracted from the continuous EEG recordings using EDIT version 4.5 software (Neuroscan Inc., Herndon, VA, USA); the average epoched signal was baseline corrected by subtracting the amplitude of EEG activity across a 500- ms pre-stimulus interval from each aggregate time point. Prior to aggregation, epochs were corrected for eye movements using the algorithm developed by Semlitsch et al. (1986), as implemented within the EDIT software. The segmented and eyeblink-corrected EEG data were then imported into Matlab (Mathworks, Inc., Natick, MA, USA) for subsequent processing, including downsampling to 128 Hz using the MATLAB resample command, which applies a low-pass anti-aliasing filter before downsampling. Trials with eyeblinks, eye movements, or muscle potentials exceeding 75 μV at any electrode were excluded from averaging.
Following artifact rejection, any ERP average waveform with fewer than three epochs per condition was flagged as missing (Shannon et al., Reference Shannon, Patrick, Venables and He2013). Three of the 253 subjects who completed the BR task were dropped from the dataset used for imputation for having insufficient (i.e., fewer than 3) artifact-free trials, while the remaining 250 subjects had a high average number of artifact-free trials (M = 68.53, SD = 9.13, range = 3–72). None of the 453 subjects who completed the EStroop task had insufficient artifact-free trails, and there was a high average number of trials included in subject averages (M = 71.33, SD = 7.61, range = 12–74). Split-half reliability estimates for these ERP components were calculated prior to imputation by applying a Spearman–Brown correction (Spearman, 1910; Brown, 1910) to the correlation between even- and odd-numbered trials. Reliability was excellent for N170, rSB(453) = .93, and acceptable for P200, rSB(240) = .74. As stated above, full information maximum likelihood estimation was used to generate imputed ERP scores for all those missing one physiological indicator of AFF−, including those who did not complete the task and those with insufficient artifact-free trials for that indicator. Excluding one subject from the base sample who was missing two indicators of AFF−, these procedures result in a final analysis sample of N = 507.
1.5.1. Physiological indicators of AFF: N170 and P2
Consistent with previous electrocortical studies of facial emotion processing (e.g., Anokhin, Golosheykin, & Heath, Reference Anokhin, Golosheykin and Heath2010; Brislin & Patrick, Reference Brislin and Patrick2019; Brislin et al., Reference Brislin, Yancey, Perkins, Palumbo, Drislane, Salekin and Patrick2018; Shannon et al., Reference Shannon, Patrick, Venables and He2013), activity recorded from selected temporal–parietal and parietal scalp recording sites (i.e., electrode site P8 for N170 response, and site midline parietal [Pz] for the P2) was re-referenced offline to relevant comparison sites (i.e., the midline site [midline central parietal] for the P8 recording site, and linked mastoids for the Pz recording site). ERP-component peaks corresponding to the N170 scoring window (150–230 ms) and P2 (150–300 ms) were quantified for fearful face stimuli. For these and subsequent analyses, BR P2 was reversed such that, consistent with the remaining two indicators, larger values were indicative of lower AFF.
1.5.2. Physiological criterion measures: fear-face-P3, aversive–neutral picture-LPP, and oddball target-P3
In addition to quantifying N170 and P2 as physiological indicators, to be combined with self-report ESI scores into a multimodal index of AFF−, we also quantified three ERP variables as neurophysiological criterion measures for purposes of evaluating convergent and discriminant validity. We computed the P3 to fear faces during the E-Stroop and late LPP modulation score (aversive–neutral) during the affective picture-viewing task to serve as convergent physiological criteria measures (ns = 506). Both components were conceptually and empirically linked to affective processing deficits observed in psychopathic CA (Venables, Hall, Yancey, & Patrick, Reference Venables, Hall, Yancey and Patrick2015). P3 to fear-face stimuli was computed at the average amplitude from 300 to 600 ms after stimulus onset (Weinberg, Venables, Proudfit, & Patrick, Reference Weinberg, Venables, Proudfit and Patrick2015). Late LPP from the affective-picture-viewing task was computed for each valence category (neutral, aversive, and pleasant) from the aggregate cross-trial waveform as the average amplitude from 600 to 1000 ms after picture onset (cf. Weinberg et al., Reference Weinberg, Venables, Proudfit and Patrick2015). A difference score was then computed from the aggregate late LPP amplitude for aversive relative to neutral pictures.
The P3 response to infrequent targets during the visual oddball task served as a neurophysiological measure of discriminant validity (n = 418). This target P3 was quantified as the maximal positive-going deflection within 300–600 ms (Yancey et al., Reference Yancey, Venables, Hicks and Patrick2013) following the target infrequent stimuli. Target P3 had been specifically linked to deficits in inhibitory control problems and such types of externalizing psychopathology (e.g., disinhibition, substance use; Hicks et al., Reference Hicks, Bernat, Malone, Iacono, Patrick, Krueger and McGue2007; Yancey et al., Reference Yancey, Venables, Hicks and Patrick2013). As such, given differential associations of AFF and inhibitory control with externalizing pathology, we did not expect target P3 to be associated with the multimodal index of AFF.
1.6. Data analyses
As an initial step, correlational analyses were performed to examine the relations of scale and physiological indicators of AFF− with (a) one another, and (b) physiological and diagnostic criterion measures. Additionally, as these indicators (i.e., N170 and P2) reflect face detection/categorization and emotional encoding processes, respectively, scores for the two physiological components for fear-face stimuli were entered as simultaneous predictors of ESI-CA, to determine if associations with N170 and P2 reflect overlapping or unique processes.
Next, a multimodal index of AFF− was computed by creating an average of the z-score for each of the three indicators (i.e., ESI-CA, EStroop N170, BR P2). Estimated multimodal index scores were computed for the full sample (N = 507), and bivariate correlations (Pearson’s r) were then used to evaluate associations between AFF− and the various criterion measures described above. Specifically, we tested for convergent validity using the specific and composite antagonistic and distress disorders described above, EStroop P3, and aversive-neutral picture late LPP, as well as discriminant validity using the specific and composite fear disorders and target P3.
2. ResultsFootnote 1
2.1. Associations among AFF indicators across measurement modalities
Results from bivariate (rs) and regression (βs, multiple Rs) among ESI-CA and hypothesized physiological indicators of AFF− are presented in Table 1 (for scatterplots, see Figure 1). As expected, associations between scale-based and physiological indicators of AFF were significant, albeit small (rs = 0.16s, for E-Stroop N170 and reversed BR P2, ps < .01), indicating that low AFF, as measured via questionnaire, was associated with a blunting of both ERPs. The corresponding r for the physiological indicators with one another was.29 (p < .001).
Table 1. Bivariate and regression analyses among AFF− indicators

Note. Correlation between N170 and BR P2: r = .29, p < .001. Regression analyses considered N170 and P2 as simultaneous predictors of ESI-CA.
a BR P2 was reversed such that, consistent with the remaining two indicators, larger values were indicative of lower affiliation.
*p < .001; † p < .01.
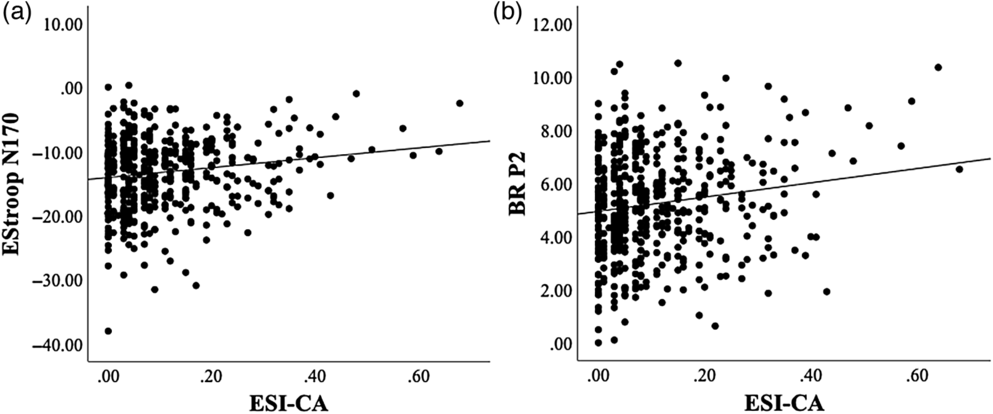
Figure 1. Bivariate associations between ESI-CA and (a) EStroop N170 and (b) BR P2 (reversed).
As described above, regression analyses were performed to examine the unique and shared predictive effects of the physiological indicators. Consistent with previous reports (Brislin et al., Reference Brislin, Yancey, Perkins, Palumbo, Drislane, Salekin and Patrick2018), analyses revealed a slight reduction in observed associations for each (βs = 0.12 and 0.12 for N170 and P2, respectively), but with residual independent prediction maintained in each case (ps < 0.01). These results suggest some overlap, but also unique contributions of processes indexed by these two early ERP components in the prediction of AFF−, i.e., face detection/categorization for N170 and emotional encoding for P2.
2.2. Operationalization and validity of multimodal measure of CA
As described above, a unit-weighted composite was used to integrate the ESI-CA trait scale measure and the two ERP indicators to index a multimodal dispositional dimension of AFF−. Table 2 shows the results from convergent and discriminant validity analyses focusing on associations of the multimodal AFF− index with diagnostic symptom counts and composite clinical dimension scores of antagonistic externalizing, fear, and distress pathology (upper portion), as well as physiological criterion variables (lower portion). As a comparative referent for evaluating the validity of the multimodal index, Table 2 also includes bivariate correlations for the constituent AFF− measures – ESI-CA, BR P2, and EStroop N170 – with the clinical symptom and physiological criterion measures.
Table 2. Bivariate correlations of each indicator and AFF− composite with physiological and diagnostic criterion variables
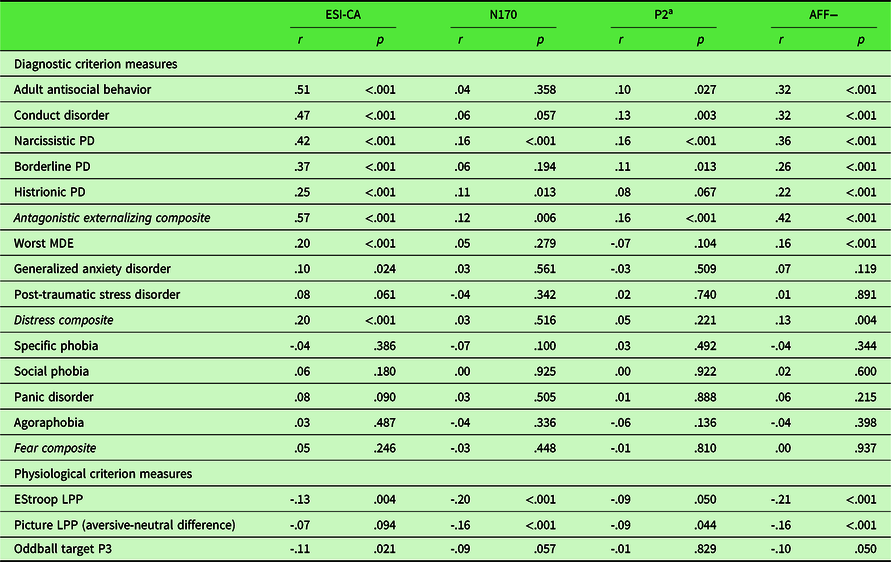
PD, personality disorder.
Note. Due to missing interview data for some clinical symptom counts, Ns range from 418 to 507.
a P2 has been reversed to be consistent with the two AFF− indicators, such that larger values are indicative of lower affiliation.
As expected, analyses demonstrated robust positive associations between ESI-CA and each antagonistic externalizing symptom variable and the composite dimension (individual rs = .25 to .51, composite r = .57, ps < .001), weak positive associations with GAD, MDE, and the distress composite (GAD, r = .10, p < .05; MDE and distress composite, rs = .20 and .20, respectively, ps < .001), and negligible associations with the fear disorders (individual rs = .03 to .08, ps = .49 to .09; composite r = .03, p = .25). ESI-CA evidenced a small negative association with EStroop LPP (r = −.13, p < .01) and with oddball target P3 (r = −.11, p < .05). Physiological indicators of AFF− showed relatively weak to null associations with clinical symptom counts. Specifically, N170 was weakly positively associated with NPD and HPD (rs = .16 and .11, ps < .001 and < .05, respectively), and P2 (reversed) showed a weak positive relationship with CD, NPD (rs = .13 and .16, ps < .01 and < .001, respectively), and BPD (r = .11, p < .05). Both physiological indicators of AFF− also evidenced small but significant positive associations with the antagonistic externalizing factor (rs = .12 and .16 for N170 and P2, ps < .01 and < .001, respectively). Additionally, N170 was negatively correlated with EStroop LPP – both were derived from the same task – and with aversive modulation of the picture-viewing late LPP (rs = −.23 and .15, respectively, ps < .001). P2 (reversed) was negatively associated with aversive modulation of the picture-viewing late LPP (r = −.10, p < .05). Of note, neither physiological variable was correlated significantly with oddball target P3 response (rs = −.09 and −.01, ps = .06 and .83, respectively).
Correlations of multimodal AFF− composite scores with diagnostic symptom counts comprising the antagonistic externalizing factor were moderate in magnitude (rs = .22 to .36, ps < .001); the association with the composite antagonistic externalizing dimension was .42 (p < .001). Further, AFF− was weakly positively associated with MDE (r = .16, p < .01), with a negligible association for GAD and PTSD (rs = .07 and .01, ps = .12 and .89, respectively) and demonstrated a weak but significant association with scores on the distress-symptom factor (r = .13, p < .01). By contrast, fear disorders were unrelated to AFF−, with at the individual-disorder level (rs = .02–.06, ps .60–.22), or the level of the composite fear dimension (r = .00, p = .94). Further analyses revealed robust negative associations for multimodal AFF− scores with theoretically relevant physiological indicators (i.e., EStroop LPP r = −.22 and aversive modulation of picture-viewing late LPP r = −.16, ps < .001). The correlation of AFF− with the target P3, used as a measure of discriminant validity, was negligible (r = −.10, p = .05).
3. Discussion
The present study was undertaken to create and validate a composite measure of AFF, incorporating self-report scale and neural-response indicators, as a step toward establishing a multimodal measurement model for this NB trait. The neural indicators consisted of two distinct early brain-ERPs to fearful face stimuli – the N170 and the P2 – from two different task procedures. These two ERP indicators each covaried significantly with a scale measure of CA (i.e., callous–aggression index of the ESI; Krueger et al., Reference Krueger, Markon, Patrick, Benning and Kramer2007), as well as with each other, supporting their use as indicators of a common dispositional construct.
A composite score was created by averaging standardized (z) scores for the two ERP indicators and the scale indicator, such that each contributed equally to the variance quantified in the NB AFF− index. Scores on this multimodal measure of AFF− converged robustly with criterion measures of affective-physiological reactivity (i.e., brain-LPP response to fearful faces and aversive pictorial images) and demonstrated transdiagnostic utility via associations with specific dimensional clinical criteria (i.e., diagnostic symptom counts) and aggregate symptom scores corresponding to the broad Antagonistic Externalizing and Distress dimensions of HiTOP. Taken together, consistent with expectations, AFF− appears to quantify the presence of a common social-dysfunction factor contributing to both internalizing and externalizing clinical problems via social withdrawal, lack of attachment and isolation, and low mood (e.g., Gao & Zhang, Reference Gao and Zhang2016).
Importantly, AFF− scores also showed clear discriminant validity in terms of nonsignificant associations with symptoms of fear disorders (specific and social phobias, panic disorder, agoraphobia) and amplitude of target-P3 brain response, a well-established neural indicator of general externalizing proneness (disinhibition; Costa et al., Reference Costa, Bauer, Kuperman, Porjesz, O’Connor, Hesselbrock and Begleiter2000; Iacono, Carlson, Malone, & McGue, Reference Iacono, Carlson, Malone and McGue2002; Patrick et al., Reference Patrick, Bernat, Malone, Iacono, Krueger and McGue2006; Yancey et al., Reference Yancey, Venables, Hicks and Patrick2013). These findings suggest conceptual and empirical separation of AFF from threat sensitivity and inhibitory control (Nelson, Strickland, Krueger, Arbisi, & Patrick, Reference Nelson, Strickland, Krueger, Arbisi and Patrick2016; Patrick, Venables et al., Reference Patrick, Venables, Yancey, Hicks, Nelson and Kramer2013; Venables et al., Reference Venables, Hall, Yancey and Patrick2015), in relation to both neural systems and clinical outcomes. Given that psychometric measures of CA (i.e., AFF−) are often moderately correlated with disinhibition, the current results (i.e., non-significant associations with target P3) suggest that quantifying AFF− psychoneurometrically shifts the dimension of CA into a distinct vector location, more consistent with the broader construct of AFF. However, further research is needed to confirm such a distinction.
Taken together, the foregoing results (a) provide further evidence that core RDoC “process” constructs (see Patrick & Hajcak, Reference Patrick and Hajcak2016) can be operationalized as NB-trait dimensions (e.g., (Nelson et al., Reference Nelson, Strickland, Krueger, Arbisi and Patrick2016; Patrick, Venables et al., Reference Patrick, Venables, Yancey, Hicks, Nelson and Kramer2013; Venables et al., Reference Venables, Hall, Yancey and Patrick2015; Yancey et al., Reference Yancey, Venables and Patrick2016), (b) support the utility of the psychoneurometric approach (i.e., integration of multiple modes of measurement) as a means for interfacing clinically relevant affiliative deficits with neurobiological indices of socioemotional information processing, and c) suggest that multimodal trait measures can serve as effective vehicles for linking empirically delineated dimensions of psychopathology with neurobiological systems and processes (e.g., Nelson et al., Reference Nelson, Strickland, Krueger, Arbisi and Patrick2016). These points are considered further below, followed by a discussion of current study limitations and directions for future research.
3.1. AFF as a transdiagnostic process
The current study extends previously published work (Patrick, Venables, et al., Reference Patrick, Venables, Yancey, Hicks, Nelson and Kramer2013; Venables et al., Reference Venables, Hall, Yancey and Patrick2015; Yancey et al., Reference Yancey, Venables and Patrick2016) and provides an bridge between an important biobehavioral construct from the RDoC framework (i.e., social affiliation; Insel et al., 2010; Cuthbert & Insel, 2013) and major dimensions of the HiTOP psychopathology model (i.e., antagonistic externalizing, distress; Kotov et al., Reference Kotov, Krueger, Watson, Achenbach, Althoff, Bagby and Zimmerman2017; Krueger et al., Reference Krueger, Kotov, Watson, Forbes, Eaton, Ruggero and Zimmermann2018). With the recent emphasis on transdiagnostic research, there is a need for a coherent approach to linking dimensions of psychopathology to neural systems (Latzman et al., in press; Perkins et al., Reference Perkins, Joyner, Patrick, Bartholow, Latzman, DeYoung, Kotov, Reininghaus, Cooper, Afzali, Docherty, Dretsch, Eaton, Goghari, Haltigan, Krueger, Martin, Michelini, Ruocco and Zald2020), and multimodal measurement models for key NB traits can facilitate progress in this regard.
The current study developed a psychoneurometric index of low AFF that demonstrated transdiagnostic utility for predicting clinical problems, both at the disorder level and at a broader dimensional level, vis-a-vis the HiTOP system. Specifically, low AFF was associated with individual externalizing disorders and with scores on a composite of these disorders. It was also associated with lifetime MDE symptomatology (i.e., worst-episode symptoms) and scores on a distress–internalizing composite. AFF− thus appears to be tapping common underlying domain-level processes at the higher order spectra level of the HiTOP system, indicating synchrony across transdiagnostic models (i.e., HiTOP and RDoC). While the associations with distress-based symptomatology in the current study were smaller in magnitude, results nonetheless highlight the transdiagnostic utility of AFF and encourage further investigation of moderating factors contributing to divergent, multifinal developmental trajectories.
3.2. Limitations and future directions
Some notable limitations of the present study must be acknowledged, which highlight important directions for future research. First, the current work was limited by the availability of psychometric measures of AFF. While CA proved useful as an indicator, it represents only one facet of the domain of social affiliation. Similarly, the physiological indicators used in the current study were somewhat circumscribed – each consisting of electrocortical responses to fearful faces. Further research is needed to identify and integrate other indicators and modes of measurement (e.g., behavioral performance, neuroimaging, and informant reports) into the initial model described here. Work of this kind would serve to iteratively broaden the nomological net of the AFF− construct and contribute to refinement of its conceptualization and measurement.
The present study was also limited in the breadth of psychopathology considered (i.e., fear, distress, and antagonistic externalizing). As AFF has been implicated as a core transdiagnostic process in various forms of psychopathology, it will be important in future research on AFF− to examine its associations with other conditions marked by social disaffiliation (e.g., psychosis, autism spectrum disorders). Additionally, the fact that observed associations for psychoneurometric AFF− with externalizing and distress disorders did not differ significantly from associations observed for the scale measure alone (i.e., ESI-CA) might be seen as a limitation. However, simple bivariate mapping among biological (e.g., neural responses) and psychological constructs is unfailingly small and inconsistent due to method variance and theoretical discrepancies between measured attributes within each measurement modality (Patrick et al., Reference Patrick, Iacono and Venables2019). Thus, the psychoneurometric approach enhances reliability and specificity of measurement by isolating common construct-related variance and omitting method variance. Importantly, the major purpose of operationalizing NB traits like AFF psychoneurometrically is not to enhance diagnostic prediction, but to broaden their nomological networks and refine how they are conceptualized (Patrick et al., Reference Patrick, Iacono and Venables2019). By incorporating neurophysiological measures into assessments of psychopathology-relevant traits, power is enhanced to identify other neurobiological indicators and characterize their interrelations and the basis of their associations with clinical outcomes. In addition, integrative assessments are likely to have clinical utility for identifying individuals with greater proclivity for risk behaviors, to be targeted for specialized intervention and prevention efforts (Mullins-Sweatt et al., Reference Mullins-Sweatt, Hopwood, Chmielewski, Meyer, Min, Helle and Walgren2020)
Finally, due to the cross-sectional nature of the data, the current study was unable to address whether low AFF constitutes a liability for the development of psychopathology, or rather an outcome of such problems. Prior evidence suggests that psychoneurometric measures effectively capture heritable variance in common between traits and clinical conditions (Venables et al., Reference Venables, Hicks, Yancey, Kramer, Nelson, Strickland and Patrick2017), but longitudinal work is also needed to establish that traits predate and contribute to the emergence of psychopathology (Perkins et al., Reference Perkins, Joyner, Patrick, Bartholow, Latzman, DeYoung, Kotov, Reininghaus, Cooper, Afzali, Docherty, Dretsch, Eaton, Goghari, Haltigan, Krueger, Martin, Michelini, Ruocco and Zald2020). Future research should capitalize on longitudinal datasets and initiatives to investigate long-term developmental trajectories of AFF− and other dispositional tendencies, beginning in early childhood. The current study represents an important first step toward understanding this trait as a risk factor for psychopathology that can help to guide hypotheses, participant selection, and choice of predictor and criterion variables in such longitudinal developmental studies.
These limitations notwithstanding the current study drew successfully on prior research establishing robust physiological correlates of CA (Brislin & Patrick, Reference Brislin and Patrick2019; Brislin et al., Reference Brislin, Yancey, Perkins, Palumbo, Drislane, Salekin and Patrick2018) to demonstrate that a trait corresponding to the RDoC construct of social affiliation can be quantified in joint self-report/neurophysiological terms, and show robust associations with both clinical and physiological criterion measures. Moving forward from here, future research should incorporate developmentally sensitive report-based and neural measures to consider the way in which psychoneurometric AFF− can refine assessment of deficits in AFF across development, beyond current report-based diagnostic methods.
Acknowledgments
Work on this article (by authors in parentheses) was supported by grants as follows: T32-AA007477 from the National Institute on Alcohol Abuse and Alcoholism (Sarah J. Brislin); W911NF-14-1-0018 from the US Army (Christopher J. Patrick); and F31-MH122096 and T32-MH93311-08 from the National Institute of Mental Health (Emily R. Perkins). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the US Government, Department of Defense, Department of the Army, Department of Veterans Affairs, or US Recruiting Command.
Conflicts of interest
None of the authors report any conflicts of interest.





