Introduction
Giardia lamblia (G. lamblia), also called G. duodenalis or G. intestinalis, is a microaerophilic, flagellated, parasitic protozoan of man and animals with a worldwide distribution that causes intestinal disorders (Lee et al., Reference Lee, Han, Ryu, Chae, Chae, Yu, Park, Park, Kim and Choi2018). G iardia lamblia has two life stages; the trophozoite and cyst. Giardiasis is transmitted by cyst form, while the trophozoite form causes acute or chronic diarrhoea, abdominal pain, malabsorption and weight (Halliez and Buret, Reference Halliez and Buret2013; Zhao et al., Reference Zhao, Haiju, Wang, Zhao and Chen2014). Children and infants are high-risk population, also in immune-compromised patients; the disease can result in morbidity and conducting mortality (Tůmová et al., Reference Tůmová, Mazánek, Lecová, Dluhošová, Typovská, Kotrašová, Ticháčková and Nohýnková2018). It is estimated that about 300 million people are affected by the giardiasis all over the world every year and the prevalence of giardiasis is further in poor hygiene regions (Feng and Xiao, Reference Feng and Xiao2011). Metronidazole, tinidazole, quinacrine, albendazole, furazolidone and paromomycin are recommended medications for giardiasis (Ordóñez-Mena et al., Reference Ordóñez-Mena, McCarthy and Fanshawe2017). However, the mentioned chemotherapy drugs exhibit adverse side effects, including gastrointestinal disturbances, nausea, vomiting, metallic taste, headache, leukopenia, mutagenic and carcinogenic in human (Harris et al., Reference Harris, Plummer and Lloyd2001). To reduce the risks of using chemical drugs, the World Health Organization (WHO) has proposed the use of medicinal plants and other natural products and reports that approximately 80% of the world population use traditional remedies for health care (Anquez-Traxler, Reference Anquez-Traxler2011). Up to now, ethnobotanical and ethnopharmacological investigations disclose a variety of natural products used for the treatment of Giardiasis which has had a variety of effects (Rahimi-Esboei et al., Reference Rahimi Esboei, Ebrahimzadeh, Gholami and Fallah Omrani2013a, Reference Rahimi-Esboei, Fakhar, Chabra and Hosseini2013b; Derda and Hadaś, Reference Derda and Hadaś2014; Méabed et al., Reference Méabed, Abou-Sreea and Roby2018).
Chitosan is a partially or fully deacetylated chitin; which exists in crustacean shells, insects' cuticles and fungi cell walls (Yarahmadi et al., Reference Yarahmadi, Fakhar, Ebrahimzadeh, Chabra and Rahimi-Esboei2016). Chitosan is biodegradable, biocompatible and non-toxic in mammalian cells (Kean and Thanou, Reference Kean and Thanou2010) and its antifungal, antibacterial and also antiparasitic activity has been proved in many studies (Tayel et al., Reference Tayel, Moussa, el-Tras, Knittel and Opwis2010; Rahimi-Esboei et al., Reference Rahimi Esboei, Ebrahimzadeh, Gholami and Fallah Omrani2013a, Reference Rahimi-Esboei, Fakhar, Chabra and Hosseini2013b; Guo et al., Reference Guo, Sun, Yin, Wang, Li, Zhou, Zhou, He and Cong2018). Many pioneer investigations indicated that considering fungi as a source of chitosan over the other traditional sources such as shrimps and non-organic source has advantageous benefits including easy access, low cost, independence of seasonal changes and safer for biomedical and healthcare applications (Ebrahimzadeh et al., Reference Ebrahimzadeh, Chabra, Gharaei-Fathabad and Pourmorad2013; Naghdi et al., Reference Naghdi, Zamani and Karimi2014). Today a growing interest has been raised in the application of nanoparticles into anti-parasite researches.
Nanoparticles as a new form of material with outstanding biological properties and low toxicity seem that there is a high potential in crossing the physiological barrier of the body access to specific target tissues (Jahangiri and Barghi, Reference Jahangiri and Barghi2018). Nanoparticles have high access to the nanoscale materials, and their toxicity is much less than the average toxic dose and acute liver damage (Jahangiri and Barghi, Reference Jahangiri and Barghi2018). These results suggest the ability of nanoparticles to target different cells for drug delivery, genetic material and diagnostic factors (Wim et al., Reference Wim and Borm2008).
Rhamnus cathartica L. belongs to Rhamnaceae family and native in Europe, western Asia, north of America and Iran (Hamed et al., Reference Hamed, Refahy and Abdel-aziz2015). The Rhamnus genus consists of anthraquinone (Emodin, Rhein, Aloe-emodin, frangulin and glucofrangulin, chrysophanol, and physcion), flavonols (catharticin, kaempferol and quercetin), anthrone rhamnoside, napthalenic derivatives, flavanol glycosides (kaempferol, rhamnazin, rhamnetin, rhamnocitrin), steroids (stigmasterol, p-sitosterol). Emodin as one of the internal combinations of R. cathartica has been shown that can inhibit the activity of both monoamine oxidase and tyrosine kinase, which was reported to have antimicrobial and antineoplastic activity (Kosalec et al., Reference Kosalec, Kremer, Locatelli, Epifano, Genovese, Carlucci, Randi and ZovkoKoncic2013; Hamed et al., Reference Hamed, Refahy and Abdel-aziz2015). Emodin revealed remarkable anti-bacterial effects against S. aureus; and even better against the standard neomycin, which is a reference antibacterial agent (Chukwujekwu et al., Reference Chukwujekwu, Coombes, Mulholland and van Staden2006). For the purposes of finding new giardiacidal agents with minimal complications, it is important to design the most promising new drugs. Current study was aimed to evaluate antigiardial activities of various concentrations of fungal chitosan isolated from Penicillium chrysogenum (P. chrysogenum), Penicillium pinophilum (P. pinophilum) and Penicillium rubefaciens (P. rubefaciens) in scale of nano, emodin delivered from R. cathartica and methanolic extraction of R. cathartica in comparison to metronidazole and furazolidone against G. lamblia trophozoites and cysts in the murine model (Balb/c mice).
Materials and methods
Chemicals supply
Emodin, quercetin, gallic acid standards, penicillin and streptomycin were purchased from Sigma-Aldrich (St. Louis, MO, USA). TYI-S-33 medium and fetal calf serum (FCS) from Gibco (India), other reagents were prepared from Merck (Germany) and Iran companies. The Penicillium species were obtained from Pasteur Institute of Iran.
Purification of G. lamblia cysts
G iardia lamblia cysts were isolated from stool samples of patients who are suffering from giardiasis and all samples were processed ordinarily within 48 h after excretion. A highly purified cyst suspension was achieved by the sucrose 0.85 M flotation method with a simplified sucrose gradient method that is previously described (Elmi T et al., Reference Elmi, Gholami, Rahimi-Esboei, Garaili, Najm and Tabatabaie2017). Purified cysts were resuspended in normal saline and stored at 4 °C for a maximum of 3 days prior to use.
DNA extraction
Purified cysts were freezed (−80 °C) and thawed (+80 °C) in 10 cycles. The genomic DNA of G. lamblia was extracted using a QIAamp DNA Stool Mini Kit (Qiagen, Germany) according to the instructions of the manufacturer. DNA samples were preserved at −20 °C until used.
PCR amplification and RFLP assay
The amplification of the gdh gene was performed with a forward primer (5′-TCA ACG TCA ACC GCG GCT TCC GT-3′) and reverse primer (5′-GTT GTC CTT GCA CAT CTC C-3′) as described by Babaei Z et al. (Reference Babaei, Oormazdi, Akhlaghi, Rezaie, Razmjou, Soltani- Arabshahi, Meamar and Hadighi2008). The PCR mixture comprised 1 µl genomic DNA, 15 µl of PCR master mix (Amplicone, UK), 1.5 µl of each primer and 11 µl distilled water. The reactions were performed in 30 µl volumes. The DNA was amplified using BioRad Thermal Cycler under the following condition: one cycle of 94 °C for 3 min, 56 °C for 1 min and 72 °C for 2 min, followed by 35 cycles, 94 °C for 1 min, 56 °C for 20 s and 72 °C for 45 s. A final extension of 72 °C for 7 min and a 20 °C hold was used. Distilled water was used as a negative control. The PCR products were electrophoresed on Safe stain 1% (W/V) agarose gel. The positive control was gathered from by the Tehran University of Medical Sciences that was isolated from human sources, and distilled water was used as anegative control. For RFLP assay, 0.5 unit of BspLI (Fermentase, Canada) and 15 µl of PCR product were used for digestion and the restriction fragments were separated in 2% agarose gels with ethidium bromide staining (Babaei et al., Reference Babaei, Oormazdi, Akhlaghi, Rezaie, Razmjou, Soltani- Arabshahi, Meamar and Hadighi2008).
Preparation of chitosan
Penicillium chrysogenum (PFCC NO: 231-3), P. pinophilum (PFCC NO:51681) and P. rubefaciens (PFCC NO:236-32) were used as a source of fungal chitosan. The fungal chitin was deacetylated by a modified method as previously described (18). After cultivation of fungal mycelia, the dry biomass was suspended with NaOH solution (1 M) and autoclaved at 121 °C for 20 min. The alkaline undissolved fractions were collected by centrifugation at 12 000 rpm for 20 min, washed with distilled water and recentrifuged to obtain pH = 7. The insoluble particles suspension was left in 2% acetic acid at 95 °C for 8 h, centrifuged, then the supernatant solution was neutralized with NaOH (2 M), the solution centrifuged and the precipitated chitosan was washed with distilled water. The degree of deacetylation (DD) of the prepared chitosan samples was determined by an acid–base titration technique. In this technique, 400 mg of prepared chitosan samples were melted in 40 mL of 0.1 M HCl at 20 °C with stirring, and then methyl orange indicator was added drop to drop (2–3 drops) to the solution and the final solution was titrated by 0.1 M NaOH. At the last stage of the titration, the colour of the solution changed from pink to orange-yellow, which was confirmed by pH meter. Five hundred grams of prepared chitosan samples were heated at 105 °C until a constant weight was reached to show the water content, and the number of free NH2 groups in the prepared chitosan samples was calculated as follows:
Molecular weight determination
The average MW of the fungal chitosan samples were determined by viscometric method. Three concentrations (10, 50 and 100 µg mL−1) of fungal chitosan solutions were prepared using the solvent system of 0.1 M acetic acid and 0.2 M NaCl (1:1, v/v). Viscosity was examined using an automatic system ubbelohde capillary type viscometer, which allows the reading of flow times of the sample taken automatically without using stopwatch in triplicate at 25 °C. The intrinsic viscosity [η] was calculated using the following formula:
(Mv) and [η] were determined the viscosity and intrinsic viscosity, respectively. (K) and (a) are constants of the values that depend on the nature of polymers and solvents.
Preparation and characterization of nanoparticles
Chitosan nanoparticle is prepared using ionic gelation method (Paresh et al., Reference Paresh, Patel, Patel and Patel2011). Different molecular weights of chitosan were melted in acetic acid (1%) and stirred overnight at 25 °C. Tripolyphosphate (TPP) and double-distilled water were mixed together to reach a concentration of 0.05% w/v and the solution was added to chitosan nanoparticle (1:5 ratio) using an insulin syringe and was stirred at 25 °C. Ultrasonication was used for 5 min to diminish the size nanoparticles.
Photon correlation spectroscopy of the chitosan nanoparticles was performed by a Dynamic Light Scattering (DLS) instrument (Nano-ZS; Malvern, UK) to determine the size profile of the preparation including zeta average size, zeta potential and polydispersity index (PDI). Size of the nanoparticles further confirmed by SEM (Seron Technology, AIS2100, Babol Noshirvani University of Technology, Mazandaran, Iran).
Plant collection and extraction
The bark of R. cathartica was collected from the forest area of Mazandaran Province, northern Iran, 1500 m above sea level during November 2017. The plant was botanically authenticated by Prof Mohammad Azadbakht (Department of Pharmacognosy and Biotechnology, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran) and deposited in the herbarium (herbarium NO: E1-212-111) of the Faculty of Pharmacy, Mazandaran University of Medical Sciences (Sari, Iran).
The crushed and air-dried bark were extracted by maceration in methanol (5 × 3 and 3 × 1 L, respectively) for 72 h at room temperature and away from light, then filtered with the filter paper and dried in a rotary evaporator with bath at 40 °C to obtain a crude extract.
Determination of total flavonoids content
The total flavonoid contents of the crude extract was determined by the aluminium chloride colorimetric method with a little modification (Sembiring et al., Reference Sembiring, Elya and Sauriasari2018). Quercetin (Sigma-Aldrich) was used to make the calibration curve. Two milligrams of the quercetin sample was added to a 25 mL volumetric flask with the solvent of 80% ethanol solution to obtain a 200 µg mL−1 stoke solution and diluted to 100, 50, 25 and 12.5 µg mL−1. A total of 0.5 mL of the prepared solutions were mixed with 1.5 mL of 95% ethanol, 0.1 mL of 10% aluminium chloride, 0.1 mL of 1 M potassium acetate and 2.8 mL of distilled water, respectively. Then the samples were incubated in room temperature for 30 min and the absorbance was measured at 415 nm. The blank sample was prepared by (the addition of the same mixture without the sample) the substitution of aluminium chloride with distilled water. Ten milligrams of the crude extracts were made with 95% ethanol solution to the volume of 10 mL in a volumetric flask and followed by the same solutions described above. The total contents of flavonoid were indicated as microgram of quercetin equivalents/g DW (μg QE/g DW). The samples analysed were conducted in triplicates.
Determination of total phenols content
Total phenolic content was determined using Folin–Ciocalteu reagent as the method adopted from Sembiring et al. (Reference Sembiring, Elya and Sauriasari2018) with slight modifications. Gallic acid (Sigma-Aldrich) was used as the standard to plot the standard curve. The extracts were prepared at the concentration of 1000 µg mL−1, and aliquots of 0.5 mL were mixed with 2.5 mL of Folin–Ciocalteu reagent (previously diluted 1:10 with deionized water) and incubated at room temperature for 10 min. Then, the sample was added by 2 mL of NaHCO3 (7.5%) and incubated at room temperature for 60 min. The absorbance of all samples was measured at 765 nm vs blank. The results are presented as gallic acid equivalents per μg of the dry weight of plant (μg GA/μg extract) and expressed as means of triplicate analysis.
DPPH free radical scavenging activity
The methodology of scavenging 1, 1-diphenyl-2-picrylhydrazyl stable free radical (DPPH) was adopted from Sembiring (2018). Briefly, 8 mg solution of DPPH was dissolved in a 100 mL volumetric flask with the solvent of methanol to obtain a concentration of 80 µg mL−1. Two millilitres of different concentrations of the extracts in methanol was added to 2 mL of prepared DPPH solution. Then the mixture was shaken and incubated for 30 min at room temperature in the dark. The absorbance was measured at 517 nm vs the blank, prepared by replacing the extract with methanol. The control was made with ascorbic acid instead of extracts at the same concentrations. The DPPH scavenging capability was calculated as follows:
where A c is the absorbance of the control and A s is the absorbance of the test compound. The IC50 value, which is the extract concentration providing 50% inhibition, was calculated as μg mL−1 from the dose–response curve. All spectrometric analyses were conducted in triplicate and averaged.
High-performance liquid chromatography analysis
The assay of extract base of the emodin was performed by the high-performance liquid chromatography (HPLC) apparatus [Knauer-YF 50-C18, 100A column (250 mm × 4.6 mm with C18 pre-column) plus quaternary gradient pump (pu-2089) plus UV-E4310 intelligent UV/vis detector, 254 nm]. The sample was eluted with isocratic mobile phase consisted of acetonitrile and water (65: 35) HPLC grade at the flow rate of 1 mL min−1 with 20 min retention time (RT).
In vitro antigiardial assay
The total extract and natural products were used with the following concentration: chitosan: 100, 200, 400 µg mL−1, nano-chitosan: 100, 200, 400 µg mL−1, emodin; 200 µg mL−1, R. cathartica: 50, 100, 200 µg mL−1, metronidazole: 400 mg mL−1, furazolidone: 250 mg mL−1, at 30, 60, 180 min. Dimethyl sulfoxide (DMSO) and phosphate-buffered saline (PBS) were considered as negative controls, otherwise metronidazole and furazolidone as positive controls.
The excystations were done according to the method described by Golami et al. (Reference Golami, Rahimi-Esboei, Mousavi, Marhaba, Youssefi and Rahimi2016). Trophozoite forms were cultured in TYI-S-33 medium enriched with bovine bile, 20% heat-inactivated FCS, penicillin (200 mg mL−1) and streptomycin (200 mg mL−1) at 37 °C in borosilicate glass tubes (PYREX®). Two millilitres of each solution and 1 × 104 washed trophozoites were added to the test tubes. The contents of the tubes were gently mixed and incubated at 37 °C for 30, 60 and 180 min. At the end of each incubation time, the upper phase was carefully removed, 1 mL of 0.1% eosin stain (MW: 691.86) were added and incubated for 15 min. The viability of were microscopically determined by counting 500 cysts.
In vivo antigiardial assay
Thirty male Balb/c mice (20 ± 2 g) at the age of at least 2 weeks were used in six groups (five mice in every group) and all animals were kept under standard environments. Stool examination ensured that mice were free from all possible protozoan infections. Mice were infected intragastrically with 1 × 103 cysts. One week after treatment, three consecutive fecal examinations were performed to prove the experimental infections. After infection, mice were treated intragastrically with fungal chitosan (10, 50, 100 µg kg−1), nano-chitosan (50 µg kg−1), emodin (100 mg kg−1), R. cathartica (50, 100, 200 mg kg−1) in comparison to metronidazole (10 mg kg−1) and furazolidone (10 mg kg−1) daily, separately. The mentioned natural products that dissolved in DMSO and 1 mL PBS were in contrast to metronidazole and furazolidone as a positive control. The negative control group received the PBS alone. After 24, 48 and 72 h of treatment, stool examinations were done to evaluate the viability of cysts. One gram of feces was homogenized in 10 cc of formal saline and the viability of cysts was done by 0.1% eosin vital staining and a haemocytometer (Dyab et al., Reference Dyab, Yones, Ibraheim and Hassan2016).
Cytotoxicity assay
Human intestinal epithelial cells (HT-29) were seeded into 96-well plates (Nunc, Roskilde, Denmark) in DMEM (100 µl) supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin except for the last row which contained only 100 µl of DMEM which was considered as the blank. Cells then were incubated at 37 °C in a humidity of 95 and 5% CO2 for 24 h. Plant extracts in concentrations of 50, 100, 200 mg mL−1 were added to each well in triplicate. After 24, 48 and 72 h of incubation time, 10 µL of MTT solution (Sigma, UK) (5 mg mL−1 in PBS) was added to each well, including controls. After 3 h of incubation at 37 °C, the supernatant was removed and 100 µL of DMSO that was purchased from Merck (Darmstadt, HE, Germany) was added. Finally, the absorbance at 570 nm was measured by a microtiter plate reader (BioTek ELX800, Winooski, VT, USA) (Lakshmi and Bai, 2016) and the viability was calculated by below formula:
Histopathological investigation
At the end of the treatment, the kidneys and livers of each animal were removed and fixed in 10% formalin. The organs sliced transversely and paraffin embedded to prepare 5 µm thick sections. The sections were stained with haematoxylin and eosin on a slide and evaluated under a light microscope and photographed by a camera microscope (Labomed, LX400) at 400x magnification. The level of liver damage was diagnosed by hepatocellular necrosis, level of inflammatory in portal area, and lymphocytic inflammatory infiltrations were scored in which grade 0 = no damage, 1 = very low level of damage, 2 = mild damage, 3 = moderate damage and 4 = severe damage. The level of kidney damage was confirmed by the survey of the glomerular inflammation, tubular necrosis and intracellular inflammation. All procedures were performed in triplicate.
Data analysis
Statistical analysis was carried out using SPSS (version 16) Software. Data were given as the mean ± standard deviation (s.d.). Paired sample t-test, one-way ANOVA and K 2 square have been done to determine the differences between groups and after treatment. P value <0.05 considers defining significant variation.
Results
Giardia lamblia genotyping
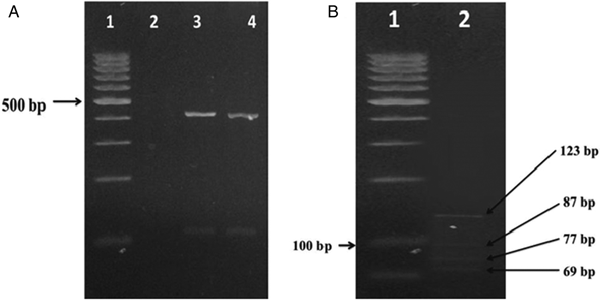
Gdh gene was amplified and digested by restriction BspLI enzyme. PCR products in the first step had a molecular weight band of 458 bp that fragment by restriction enzymes and the results revealed that the isolate is characteristic four-band pattern of assemblage AII (Fig. 1).
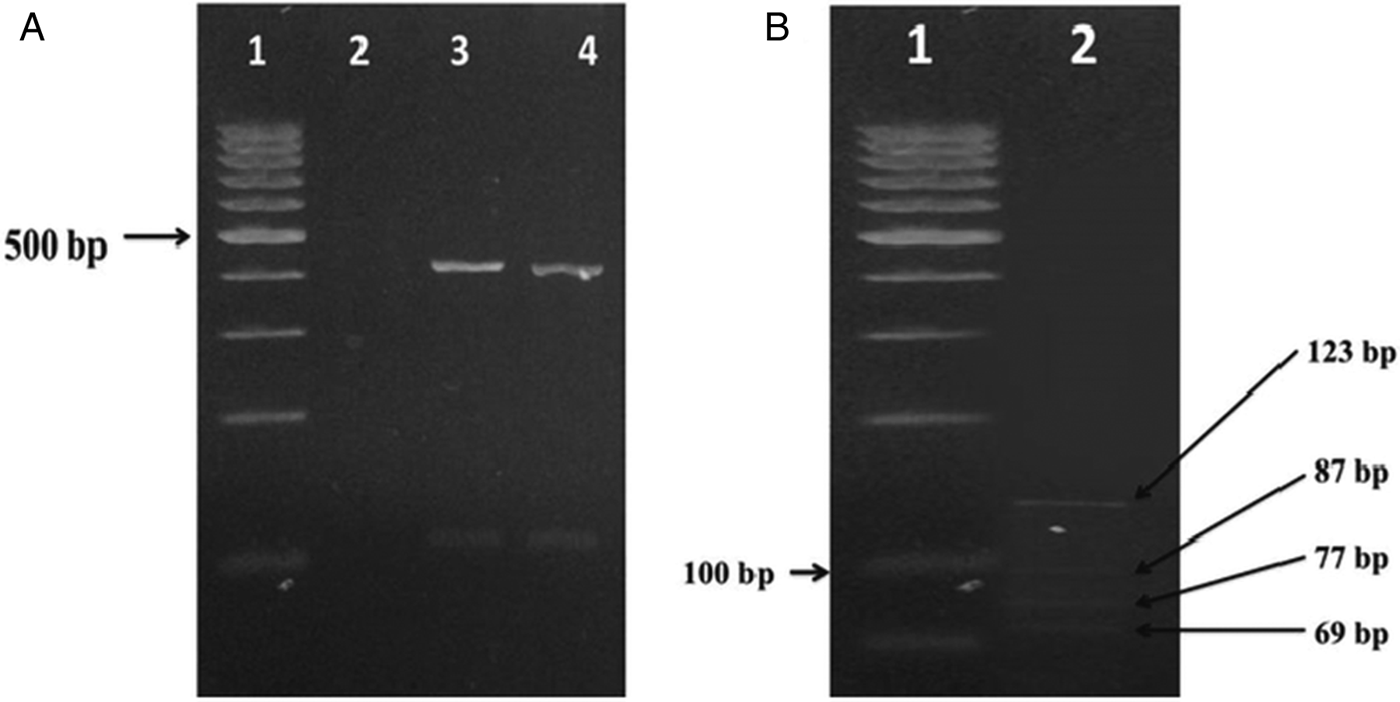
Fig. 1. (A) Electrophoretic separation of PCR product from DNA amplified at the gdh locus of G. lamblia, lane 1; 100 bp marker, lane 2; negative control (distilled water), line 3; positive control and line 4; PCR products from clinical samples. (B) Lane 1; 50 bp marker, lane 2; digestion of PCR product of G. lamblia.
Molecular weight of chitosan and DD and viscosity
The DD was achieved 75.1, 64, 57.9, viscosity 10.1, 9, 9.3 and MW 1.3 × 105, 9.3 × 104, 5.3 × 104 for P. chrysogenum, P. pinophilum, P. rubefaciens, respectively.
Morphology analysing by SEM
The particle size data of chitosan NPs by DLS showed (Fig. 2A) a size of 387 nm with a PDI of about 0.23. SEM (Fig. 2C) images show that the average size is around 300 nm with spherical morphology. Surface charge and thereby the stability of the prepared nanoparticle systems was determined by zeta potential measurements. Zeta potential value was found to be 48.7 mV (Fig. 3B). This value lies in the stable range, indicating that this nanoparticle system was stable.
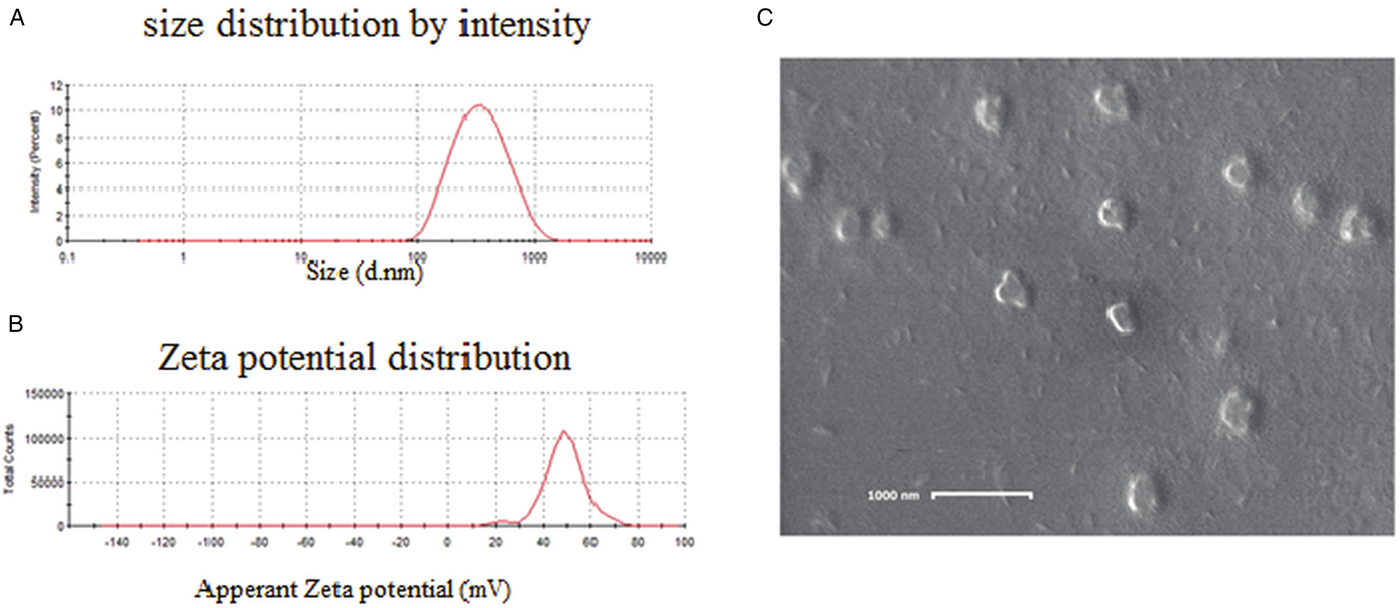
Fig. 2. (A) DLS showing the size distribution of chitosan nanoparticles; (B) zeta potential of chitosan nanoparticles showed good positive charge; (C) SEM image of chitosan nanoparticles showed proper morphology.
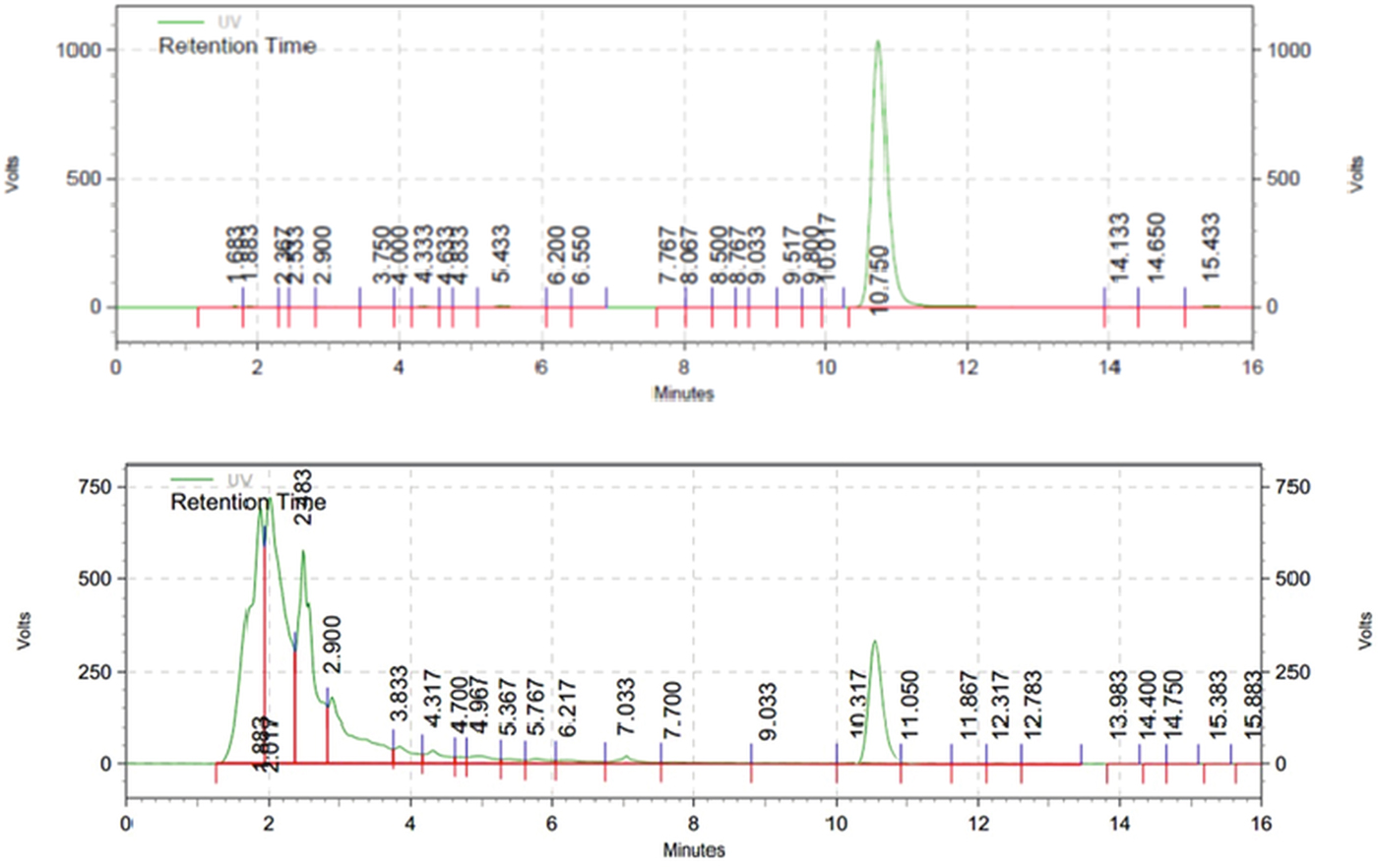
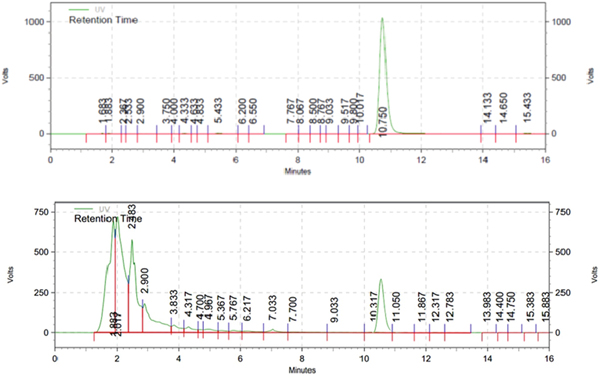
Fig. 3. HPLC chromatogram of standard emodin (up) and methanolic extract of R. cathartica (down); wavelength 254 nm; flow rate, 1 mL min−1; injection volume, 20.0 mL; concentration, 200 mm each.
Phytochemical screening
Total flavonoid and phenolic contents
The yield of extract was 17%. The total flavonoid content was 23.15 ± 1.355 mg CE/g DW in reference to the standard curve (y = 0.0063x − 0.0055, r 2 = 0.999). The total phenolic content was 351.66 ± 4.85 mg GAE/g DW in reference to the standard curve (Y = 0.0028x − 0.0362, r 2 = 0.999.).
DPPH free radical scavenging activity
The calculated IC50 value of methanolic extract was 74.46 ± 1.89 µg mL−1 while for positive control ascorbic acid it was 57.92 ± 2.21 µg mL−1.
HPLC separation
The isocratic HPLC chromatogram of emodin exhibited the narrower and sharp peak in 10.75 min (Fig. 2). The methanolic extract of R. cathartica reported the shorter peak in approximately the same RT in 10.31 min (Fig. 3). The similar chromatograms proved the existence of emodin in R. cathartica methanolic extract.
In vitro antigiardial activity
The mortality rate of trophozoites and cysts at the highest concentration and exposure time were (99 ± 6.3 and 96 ± 5.8) and (99 ± 2.7 and 95 ± 3.82) for common and nano form of P. chrysogenum, (100 ± 1.2 and 97 ± 6.3) and (99 ± 3.8 and 94 ± 4.32) for common and nano form of P. pinophilum, (99 ± 0.9 and 93 ± 4.5) and (100 ± 0.1 and 98 ± 6.54) for common and nano form of P. rubefaciens, (100 ± 1.1 and 95 ± 3.22) for emodin, (98 ± 2.1 and 96 ± 4.9) for R. cathartica, (100 ± 1.5 and 96 ± 4.76) for metronidazole, (97 ± 3.4 and 92 ± 3.29) for furazolidone, (2 ± 0.8 and 2 ± 0.3) for negative control. The toxicity of these treatments on trophozoites is independent to exposure time without any significant difference (P > 0.05). The mortality rate of trophozoites and cysts in treatment groups was similar to positive control (P > 0.05), otherwise significantly higher than negative control (P < 0.05). Concentration as a main variable has a significant effect on mortality rate (Tables 1 and 2). Nano-chitosan has a better effect than chitosan; however, the difference was not significant (P > 0.05).
Table 1. The mortality rate of G. lamblia trophozoite in different groups, in vitro
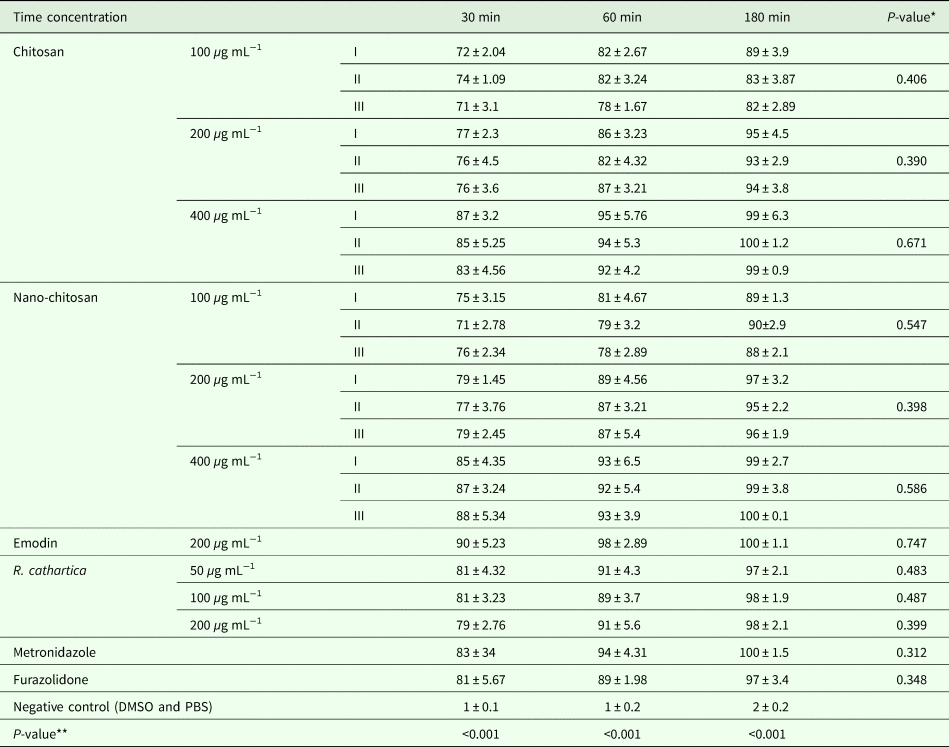
*Effect of time.
**Effect of concentration.
(I: P. chrysogenum, II: P. pinophilum, III: P. rubefaciens).
Table 2. The mortality rate of G. lamblia cyst in in different groups, in vitro
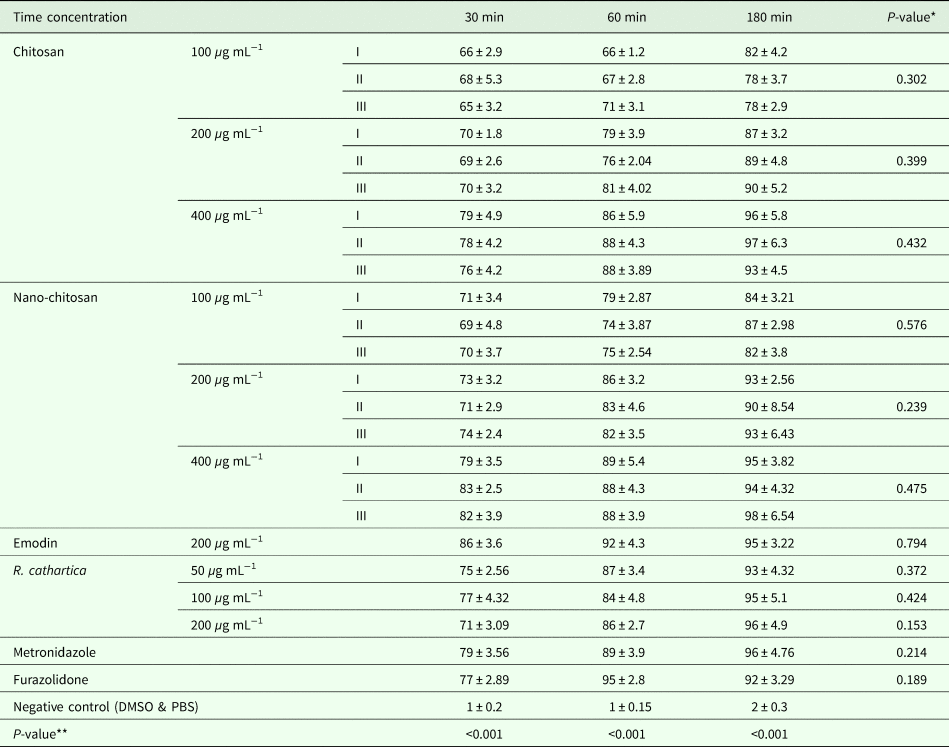
*Effect of time.
**Effect of concentration.
(I: P. chrysogenum, II: P. pinophilum, III: P. rubefaciens).
In vivo antigiardial activity
As shown in Table 3, the excretion of cysts was decreased after treatment in comparison to negative control (P < 0.05). Concentration was another important factor in this study, but in all cases there was no significant difference. Emodin as the most important contents of R. cathartica was more effective against G. lamblia than the total extract (P < 0.05).
Table 3. Mean percentage of excreted cysts before and after treatment in comparison to control groups in vivo (n = 5, mice per a group)
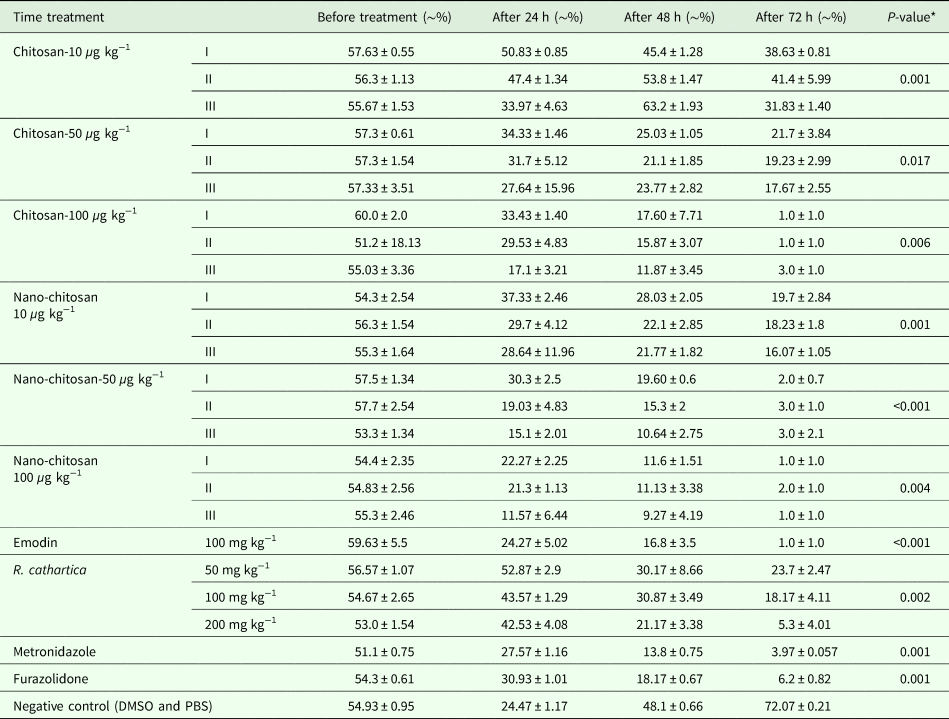
*The differences between groups before and after treatment.
(I: P. chrysogenum, II: P. pinophilum, III: P. rubefaciens).
Approximately, in most of the concentration, all fungal chitosan, nano-chitosan and emodin were more effective than metronidazole and furazolidone against G. lamblia in mice model.
The excretion of the cysts was increased by the increase of the chitosan concentration and there are significant differences among cyst excretion in the concentration of 10 and 100 µg kg−1 (P < 0.05). Of course, the time as an effective factor influenced the mortality rate of G. lamblia cysts and trophozoites; by increasing the time of exposure, the mortality rate is being increased.
Since the results of the study in different doses of chitosan show a linear relationship with the doses of the parasite, according to the linearity obtained from the regression equation (y = 32.43 + 0.73X), EC 50 of chitosan at a dose of 24 mg mL−1 was calculated. On the other hand, the linear regression equation for the extract of R. cathartica showed (y = 47.5 + 0.21X), the EC50 value is based on the line of 12 mg (Fig. 4). However, the regression equation for the nano-chitosan was not linear and the EC50 was predicted as 12 µg mL−1.

Fig. 4. (A) Determination of EC50 values of fungal chitosan on Giardia lamblia in vivo, (B) Determination of EC50 values of R. cathartica extract on Giardia lamblia in vivo.
Cytotoxicity activity
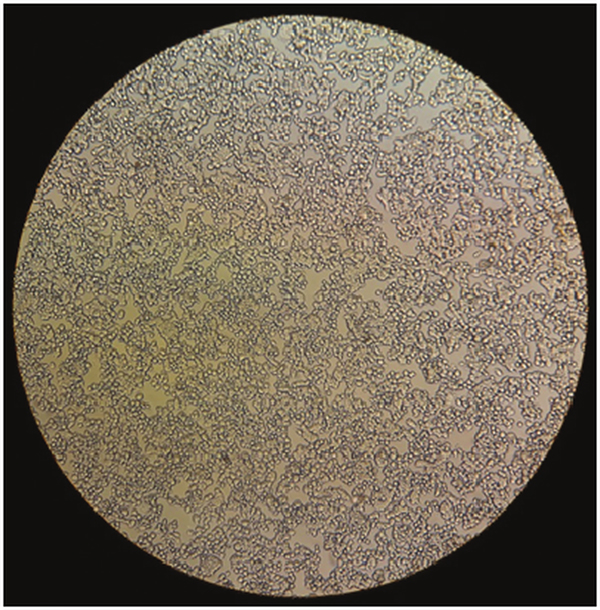
The tetrazolium-based colorimetric MTT assay indicated that fungal chitosan, nano-chitosan and R. cathartica methanolic extract in different concentrations and emodin had no toxicity effects on the HT-29 cells viability after 24, 48 and 72 h (P > 0.05) (Table 4 and Fig. 5). By prolonging exposure time, no significant toxicity has been observed in all groups (P > 0.05).
Fig. 5. Fresh HT-29 cells after treatment by Chitosan, R. cathartica and emodin.
Table 4. Cytotoxicity results of the different groups on the HT-29 cells
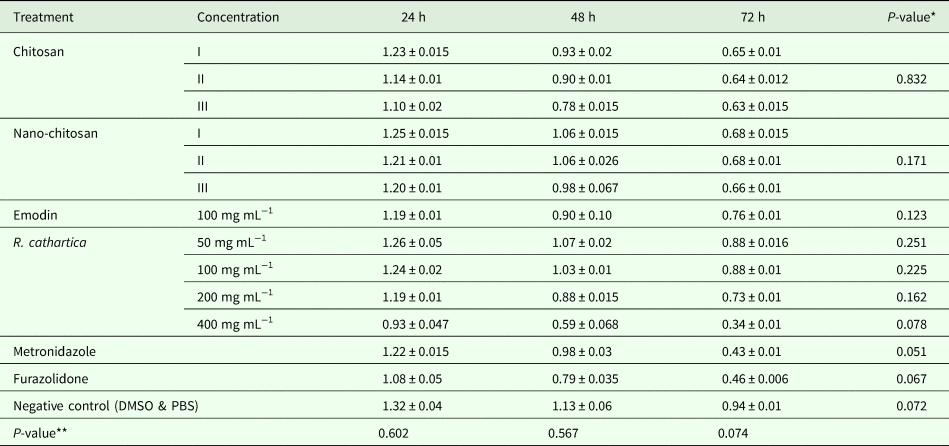
*Effect of exposure time on toxicity of drugs on normal cell line.
**The toxicity of drugs on normal cell line.
(I: P. chrysogenum, II: P. pinophilum, III: P. rubefaciens).
Histopathology examination
The histopathological examinations of the histological sections of the livers and kidneys in different groups (chitosan, nano-chitosan, emodine, R. cathartica, metronidazole and furazolidone) after 72 h of treatment were represented, respectively. No evidence of abnormality, tissue degeneration, inflammation and necrosis was existed in liver and kidney sections in all groups. Hepatocytes were normal with polygonal shape and normal sinusoidal and portal space. Kidney tissue and glomerular morphology and tubular epithelia were normal in all groups.
Discussion
In the present study, the antigiardial activity of fungal chitosan, nano-chitosan, emodin, nano-emodin and methanolic extract of R. cathartica evaluated in vivo. Natural products are valuable sources for developing new drugs over centuries (Koehn and Carter, Reference Koehn and Carter2005). Owing to the growing rate of resistance to giardiasis drugs, introduction of new agents are essential for effective treatment (Croft et al., Reference Croft, Sundar and Fairlamb2006). Up to now, the genus Rhamnus has not been studied for antigiardial effects, however there are reports of the antimicrobial effect of the species (Kosalec et al., Reference Kosalec, Kremer, Locatelli, Epifano, Genovese, Carlucci, Randi and ZovkoKoncic2013). R hamnus cathartica showed equal anti-giardial activity against trophozoites and cyst of G. lamblia in comparison to metronidazole and furazolidone as positive controls without any significant differences (P = 0.09). Flavonoids, phenolics and anthraquinones compounds in this genus are effective in antimicrobial activities and this is due to the ability to penetrate into the microorganisms cells (Sultana et al., Reference Sultana, Anwar and Przybylski2007; Qadir et al., Reference Qadir, Shahzadi, Bashir, Munir and Shahzad2017). These compounds could bind to the nucleotide-binding site of multiple drugs proteins with an associated heighten the accumulation of intracellular drug as a modulator. Several investigations have also indicated an antigiardial activity of a variety of total herbal extracts such as Artemisia annua (IC50 = 51 mg mL−1), Sambucus ebulus (IC50 = 43 mg mL−1) (Rahimi-Esboei et al., Reference Rahimi Esboei, Ebrahimzadeh, Gholami and Fallah Omrani2013a, Reference Rahimi-Esboei, Fakhar, Chabra and Hosseini2013b).
The IC50 values described in these studies variety from 0.8 to 300 µg mL−1, but the majority of plant extracts presented IC50 values more than 100 µg mL−1 (Calzada et al., Reference Calzada, Y´epez-Mulia and Aguilar2006; Barbosa et al., Reference Barbosa, Calzada and Camposb2007; Machado et al., Reference Machado, Augusto, Salgueiro, Cavaleiro, José and Do Céu Sousa2010). Emodin with anthraquinone structure is one of the main compositions of R. cathartica extract and has anti-parasitic effects; better than positive controls and common treatments in an in vivo situation (Srinivas et al., Reference Srinivas, Babykutty, Sathiadevan and Srinivas2007; Li et al., Reference Li, Song, Yin, Jia, Li, Zhou, Zou, Li, Yin, Yue, Ye, Lv, Shi and Fu2016). The reports on anthraquinones parasiticidal activities are limited and the mechanism of action is not cleared yet. Anthraquinones, especially emodin, can selectively inhibit the casein kinase II and tyrosine kinases activity (Li et al., Reference Li, Song, Yin, Jia, Li, Zhou, Zou, Li, Yin, Yue, Ye, Lv, Shi and Fu2016). Protein tyrosine kinases play significant roles in the regulation of cell growth by catalysing the transfer of phosphate from ATP to the hydroxyl of tyrosine. The conjugated double-bond system in aromatic rings with the resonant properties causes the tremendous antimicrobial effects. The resonance in anthraquinone structure creates the electron donors (HD) and acceptors leading to form hydrogen bonds with the cell membrane of microbial and leakage of the intracellular contents (Srinivas et al., Reference Srinivas, Babykutty, Sathiadevan and Srinivas2007; Li et al., Reference Li, Song, Yin, Jia, Li, Zhou, Zou, Li, Yin, Yue, Ye, Lv, Shi and Fu2016; Venkatachalam et al., Reference Venkatachalam, Girard-Valenciennes, Caro, Dufossé and Fouillaud2016). Chukwujekwu et al. (2009) reported that emodin had antibacterial effects against Bacillus subtilis and Staphylococcus aureus with Minimum Inhibitory Concentration (MIC) values of 7.8 × 10−3 and 3.9 × 10−3 mg mL−1, respectively. Emodin and three anthraquinone derivatives isolated from Rheum emodi had antimalarial activity on PfK1 strain (Palladium falciparum chloroquine resistant strain) with the IC50 of 2.28, 2.49 and 2.48 µM, respectively, and without any cytotoxicity on monkey kidney cell line (Pandeti et al., Reference Pandeti, Gunjan, Paidipelli, Tripathi and Tadigoppula2014).
Chitosan in high acetylation was more effective in comparison to the low acetylation form. Similar findings revealed the significant difference in the efficacy of fungal chitosan in comparison to untreated group in the concentration of 10 µg mL−1 against G. lamblia cysts (Yarahmadi et al., Reference Yarahmadi, Fakhar, Ebrahimzadeh, Chabra and Rahimi-Esboei2016). The modification of chitosan nanoparticles using sodium TPP by ionotropic gelation method revealed more advantages over the common form.
This modification can promote the bioavailability, specificity and sensitivity and decreasing toxicity in human body (Ebrahimzadeh et al., Reference Ebrahimzadeh, Chabra, Gharaei-Fathabad and Pourmorad2013). The SEM showed that the pure chitosan has a plain, smooth, compact and homogeneous surface without pores, whereas, chitosan nanoparticles have a rough, porous and larger exposed surface which increases adsorption. Several researchers reported that the chitosan nanoparticles have the spherical shape, solid dense cubical or rectangular structure and rough surface morphology (Wim et al., Reference Wim and Borm2008; Jahangiri et al., 2018; Wardani et al., Reference Wardani, Mahmiah and Sudjarwo2018).
Thus, the smaller the particle, the higher will be its mobility and surface interaction, resulting in enhancing the antimicrobial activity and the bioavailability of poorly water-soluble molecules and could improve the efficacy of the particle-based oral drug delivery systems (Sui et al., Reference Sui, Fang, Chi and Zhang2010; Pilon et al., Reference Pilon, Spricigo, Miranda, Moura, Assis, Mattoso and Ferreira2015; Jahangiri et al., 2018). R hamnus cathartica methanolic extract, emodin and chitosan had no cytotoxicity effects on the HT-29 cells viability after 24, 48 and 72 h. However, after many times (72 h) high deacetylated chitosan, high deacetylate nano-chitosan and R. cathartica methanolic extract in the concentration of 400 mg mL−1 reduced the cell viability to <50%.
Several in vivo and in vitro studies have confirmed the non-toxicity effect of chitosan on human cell lines, and the FDA has approved chitosan for clinical applications (Baldrick, Reference Baldrick2010). Also, it is approved for dietary applications in Japan, Italy and Finland (Kim, Reference Kim and Kim2013). Biodegradability and biocompatibility as some of their particular properties make them appropriate for medical application (Kucharska et al., Reference Kucharska, Ciechańska and Niekraszewicz2010; Kim, Reference Kim and Kim2013). Although the particular mechanism of antiprotozoal activity of chitosan is not fully explained, but the effect on the cell wall of parasites is the suggested mechanism (Tayel et al., Reference Tayel, Moussa, el-Tras, Knittel and Opwis2010; Rahimi-Esboei et al., Reference Rahimi Esboei, Ebrahimzadeh, Gholami and Fallah Omrani2013a, Reference Rahimi-Esboei, Fakhar, Chabra and Hosseini2013b).
Chitosan is more effective than the majority of other plant extracts. Additionally, the effect observed on viability revealed advantageous for the development of potential giardicidal treatments from chitosan.
Conclusion
The effects of different therapies on trophozoite have been better than cysts. But contrary to the previous mindset that we thought that trophozoite was more sensitive than cyst; the difference between cysts and trophozoites was not significant in this study. Due to differences in cellular structure and cell wall in cyst and trophozoite, this study has shown that there is no significant difference in drug effects. The chitosan derived from fungi, nano-chitosan, R. cathartica extract and emodin may be used as important compounds for the development of novel therapeutic drugs for the treatment of giardiasis. Mentioned remedies showed to be safe for mammalian cells by in vitro and in vivo tests. Also, the nano-chitosan synthesis improved the therapeutic response in lower dosage. In addition, the chitosan has been commonly available, easily prepared and low-priced. Therefore, nano-chitosan was accepted as a good candidate among natural giardiacidal agents.
Author ORCIDs
Aroona Chabra, 0000-0002-2432-0362
Acknowledgements
Special thanks to Mr. Danial Chabra (Faculty of Food Science & Industry, Gorgan Agricultural Sciences University, Gorgan, Iran) for language editing.
Financial support
This work was supported by a grant from Student Research Committee (No: 72 & 106 & 107) and Pharmaceutical Science Research Center, Faculty of Pharmacy (No: 2843), Mazandaran University of Medical Sciences, Sari, Iran.
Conflict of interest
None.
Ethical standards
The experimental protocols were approved by the ethical committee of Mazandaran University of Medical Sciences, in agreement with the guidelines for the use and care of laboratory animals and the treatment started after the initial infection.