Published online by Cambridge University Press: 24 August 2022
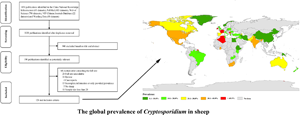
Cryptosporidium spp. are important pathogens with some species causing diarrhoea in humans and animals. Sheep are one of the most common potential hosts for various Cryptosporidium spp. The prevalence of Cryptosporidium in sheep globally was evaluated from published information including molecular data via meta-analysis. In total, 126 datasets from 41 countries were included for final quantitative analysis. Sheep aged <3 months had a significantly higher prevalence (27.8%; 3284/11 938) than those at the age of 3–12 and >12 months. The prevalence of Cryptosporidium in sheep with diarrhoea of 35.4% (844/1915) was higher than in sheep that did not show diarrhoea (11.3%; 176/1691). Fourteen Cryptosporidium species/genotypes were detected in sheep globally. The proportion of subgenotype family XIIa of Cryptosporidium ubiquitum was 90.0% (216/240); the proportions of subgenotypes IIdA20G1 and IIaA15G2R1 of Cryptosporidium parvum were 15.4% (62/402) and 19.7% (79/402). The results indicate that C. parvum is the dominant species in Europe while Cryptosporidium xiaoi is the dominant species in Oceania, Asia and Africa and C. ubiquitum is the dominant species in North America and South America. Subgenotype family IIa of C. parvum is particularly widespread among sheep worldwide. The results highlight the role of sheep as a reservoir host for zoonotic cryptosporidia and the need for further study of prevalence, transmission and control of this pathogen in sheep.