Introduction
Increasing urbanization is having negative impacts on bats and their key roosting sites. Natural bat roosting sites are uncommon but increases in ecotourism have damaged or destroyed such sites, and in some cases populations of bats have been extirpated (Beshkov, Reference Beshkov and Meine1998; Furman & Özgül, Reference Furman and Özgül2002, Reference Furman and Özgül2004; Mann et al., Reference Mann, Steidl and Dalton2002; Albayrak, Reference Albayrak and Albayrak2005; Pennisi et al., Reference Pennısı, Holland and Steın2005; Rudolph et al., Reference Rudolph, Liegl and Karataş2005; Niu et al., Reference Niu, Wang, Zhoa and Liu2007; Papadatou et al., Reference Papadatou, Butlin, Pradel and Altringham2009). Natural caves and artificial underground sites are widely used as roosting sites by bats and may be occupied by large breeding and/or hibernating populations (Altringham, Reference Altringham1996). Disturbance by visitors to caves is a widespread problem and has been documented as a major threat to cave-dwelling bats (Barbour & Davis, Reference Barbour and Davis1969; Humphrey & Kunz, Reference Humphrey and Kunz1976; Tuttle, Reference Tuttle1979; Thomas et al., Reference Thomas, Dorais and Bergeron1990; Speakman et al., Reference Speakman, Bullock, Eales and Racey1992; Wegiel & Wegiel, Reference Wegiel and Wegiel1998; Martin et al., Reference Martın, Leslıe, Payton, Puckette and Hensley2003; Pennisi et al., Reference Pennısı, Holland and Steın2005). Disturbances of bat roosts can result in the abandonment of hibernation and maternity caves (White & Seginak, Reference White and Seginak1987; Tuttle & Taylor, Reference Tuttle and Taylor1998; Ludlow & Gore, Reference Ludlow and Gore2000; Thomson, Reference Thomson2002).
Contemporary efforts for bat conservation focus on protecting caves and the various types of bat colonies they house (American Society of Mammalogists, 1992). Construction of gates at the entrances of caves is a typical method of protection for important bat roosts (Tuttle, Reference Tuttle, Aley and Rhodes1977; Humphrey, Reference Humphrey1978; Tuttle & Stevenson, Reference Tuttle, Stevenson, Zuber, Chester, Gilbert and Rhodes1978). Of all the full closure structures, gates with horizontal bars are the most commonly used. Given the paucity of published data on the effects of gates, bat activity needs to be carefully monitored before and after the placement of a gate (Pugh & Altringham, Reference Pugh and Altringham2005). In addition, illumination (Mann et al., Reference Mann, Steidl and Dalton2002) and landscaping in caves visited by tourists can negatively affect the behaviour of bats. Besides appropriate gate construction, a suitable visitor schedule and a management plan are therefore necessary to protect bats and their roots in such caves.
The distribution and availability of suitable roosting sites is a limited resource for some cave-dwelling bat species (Humphrey, Reference Humphrey1975). The often specialized physiological roosting requirements of bats dictate the environments in which a species can or cannot successfully roost. The Dupnisa Cave System is one of the largest winter and summer bat roosts in south-east Europe and the most important site for bats in the Thrace region of Turkey (Furman & Özgül, Reference Furman and Özgül2004; Paksuz, Reference Paksuz2004; Paksuz et al., Reference Paksuz, Özkan and Postawa2007). The popularity of this cave system with tourists and speleologists previously posed a serious threat to the bats that roost there.
The effects of human activities on the bat community in a region can be assessed only by continuous monitoring. Because roosts with different functions are needed to complete the annual life cycle of a species, protecting only those sites with large numbers of bats may be insufficient: management for conservation requires a comprehensive understanding of roosting ecology (Kurta & Kennedy, Reference Kurta and Kennedy2002). The aim of this study was therefore to determine seasonal numbers and roost use by bats in the Dupnisa Cave System before and after it was opened to tourism and to develop a management plan for protection of the bat species using the cave system. We also evaluated the effects of tourism and gates on the bats.
Study area
The Dupnisa Cave System lies south of Kırklareli-Sarpdere Village in the forested Yıldız (Istranca) Mountains in Thrace, the European part of Turkey. At 2,720 m long this cave system is the second longest in Thrace and is regarded as a cave system because it includes two floors and three connected caves (Fig. 1). The process of formation and development of this cave system continues. The main gallery, through which an underground stream flows, is Sulu Cave (1,977 m long). Formation of the two caves above the main gallery (Kuru Cave, 480 m; Kız Cave, 263 m) is complete (Nazik et al., Reference Nazık, Tork, Ozel, Mengı, Aksoy, Derıcı and Kutlay1998).

Fig. 1 The Dupnisa Cave System, showing the location of the three main caves, the areas open to tourists, and the gated and ungated entrances (Plate 1). Adapted from Nazik et al. (Reference Nazık, Tork, Ozel, Mengı, Aksoy, Derıcı and Kutlay1998).
This is the first cave system in Turkey to open with a visitor schedule and a gate construction based on the seasonal use of the cave system by bats, and long-term monitoring is conducted. The cave system has been visited by nearly 21,000 visitors each year since it was opened to tourism in July 2003; most of the visits occur from late May to July. Tourist circuits have been constructed in the first 200 m of the Sulu Cave and the first 230 m of the Kuru Cave but the Kız Cave is closed to tourism (Fig. 1). The cave system has four entrances. We gated the two entrances to the tourist circuits before the system was opened to tourism. The gates were constructed with horizontal iron bars that have a 200-mm spacing (Plate 1). The other entrances of the cave system, which are located away from the area opened to tourism, are difficult to access and remain ungated to minimize the negative effects of the two gates on the bats.

Plate 1 The gated entrances to (a) Sulu Cave and (b) Kuru Cave, used to control the entry of tourists. The horizontal bars have a spacing of 200 mm to allow bats to move freely.
Methods
We carried out 15 surveys before (2002–2003) and 38 surveys after (2004–2008) the cave system was opened to tourism. Surveys were conducted approximately once every 40 days for 1–2 days, during daytime, when the bats were in their roosts. In each survey we recorded the population sizes and use of roosts by each bat species in the three parts of the cave system. We could not survey in some months because of bad weather and various other unanticipated circumstances. Bats were identified visually with reference to Dietz & Von Helversen (Reference Dietz and Von Helversen2004); when necessary we collected bats with a net mounted on an extendable rod and released them after identification.
Isolated individuals and small colonies were counted directly. The sizes of larger colonies were estimated by counting bats in an area of 30×30 or 50×50 cm and multiplying the number in the area counted by the total area of the colony. For some colonies we repeated the count several times and estimated that our margin of error was c. 10%. The spatial distribution of the bats was quantified using Green's index (C x; Green, Reference Green1966), which varies between 0 (for a random distribution) and 1 (for a clumped distribution). Differences between the total population sizes of bat species in the different parts of the cave system in different seasons were examined by independent sample t-tests. Wilcoxon matched-pair signed ranks tests were used to test for differences between the population sizes in the various parts of the cave system before and after installation of gates and opening for tourism. Correlations between the seasonal use of the various parts of the cave system before and after the opening of tourism were compared with Spearman's rank correlation coefficient test. Total numbers and roost use were investigated in the winter/hibernation period (November–March) and summer/nursery period (April–October).
Results
We found 15 bat species in the cave system: Rhinolophus ferrumequinum, Rhinolophus hipposideros, Rhinolophus euryale, Rhinolophus mehelyi, Rhinolophus blasii, Myotis myotis, Myotis blythii, Myotis bechsteinii, Myotis nattereri, Myotis emarginatus, Myotis mystacinus, Myotis daubentonii, Myotis capaccinii, Plecotus auritus and Miniopterus schreibersii. The maximum numbers of bats recorded was 54,600 hibernating in January 2004 and 11,000 breeding/nursing in August 2006. The three parts of the cave system were used by different species to varying degrees, according to season.
Sulu Cave was used for hibernation by nine species: R. ferrumequinum, M. myotis, M. blythii, M. emarginatus, M. mystacinus, M. nattereri, M. capaccinii, M. daubentonii and M. schreibersii. The cave was used only for seasonal movements during the summer and not for breeding/nursing. These nine species comprised, overall, 73% of the total number of bats recorded in the cave system during the study. The most numerous species recorded in the cave in a single survey were M. schreibersii (36,000 individuals), M. blythii (4,000), R. ferrumequinum (2,200) and M. capaccinii (1,800). The highest number of bats recorded in this cave was 44,500 hibernating individuals in January 2004 (Table 1).
Table 1 Monthly numbers of bats (all 17 species combined) before (2002–2003) and after (2004–2008) tourism activities commenced in the Dupnisa Cave System (Fig. 1). A blank cell indicates the absence of a survey in that month and year.
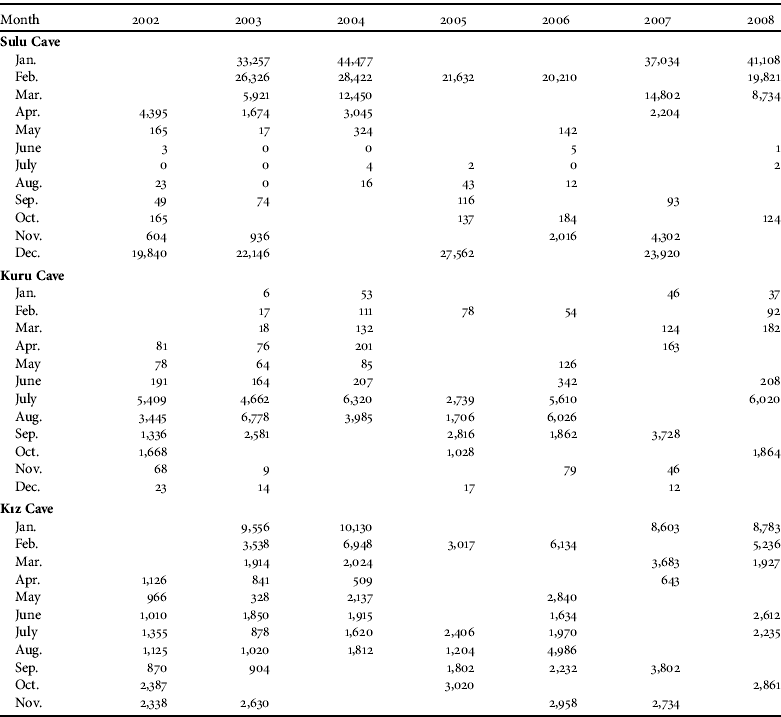
Kuru Cave was used by 10 species (R. ferrumequinum, R. hipposideros, R. euryale, R. mehelyi, M. myotis, M. blythii, M. bechsteinii, M. capaccinii, M. schreibersii and P. auritus) that, overall, comprised 7% of the total number of bats recorded in the cave system. The cave was used by bats throughout the year, with the majority of the bat assemblages being for breeding and nursing in the summer. The most numerous species in the cave in a single survey were R. euryale (2,500), M. schreibersii (2,400), M. blythii (1,200) and R. ferrumequinum (900). The maximum number of bats recorded in the cave was 6,800 breeding and nursing individuals in August 2003 (Table 1).
Kız Cave was used by seven species for hibernating and breeding/nursing throughout the whole year (R. ferrumequinum, R. euryale, R. mehelyi, R. blasii, M. myotis, M. blythii and M. schreibersii) that, overall, comprised 20% of the total number of bats recorded in the cave system. The most numerous species in the cave in a single survey were M. schreibersii (9,500), R. euryale (1,600), R. ferrumequinum (300) and R. mehelyi (300). The maximum number of bats counted was 10,100 hibernating individuals in January 2004 (Table 1).
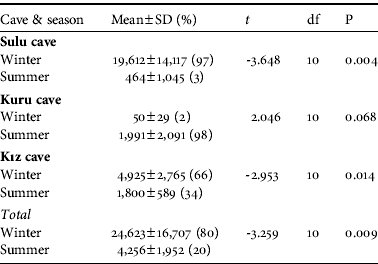
Overall, significantly more bats were recorded in the winter than in the summer (Table 2), and the bat colonies were noticeably clustered for hibernation in the winter but less so for breeding/nursing in the summer. The differences in numbers between seasons were significant in Sulu Cave and Kız Cave but not in Kuru Cave (Table 2). The spatial distribution of bats in the cave system was random in both seasons (C x=0.09 and C x=0.03 in winter and summer, respectively). The spatial distribution of bats in Sulu Cave was close to random in the winter (C x=0.10) but more clumped in the summer (C x=0.72). In Kuru and Kız Caves bats were randomly distributed in both winter and summer (Kuru: C x=0.06 and C x=0.15, respectively; Kız: C x=0.06 and C x=0.01, respectively). The highest numbers of bats recorded in the cave system in any single survey before and after the system opened to tourism were 42,800 and 54,600 hibernating, respectively, and 7,900 and 11,000 breeding/nursing, respectively. The total number of bats in the cave system was significantly lower before (2002–2003) compared to after (2004–2008) the opening for tourism (z=-2.353, P<0.05). The total number of bats in Sulu Cave (z=-1.255, P>0.05) and Kuru Cave (z=-1.334, P>0.05) were not significantly different before and after opening to tourism but the difference was significant for Kız Cave (z=-2.118, P<0.05). The correlations between the different parts of the cave system before (r s:sulu/kuru=-0.90, P<0.001; r s:sulu/kız=0.67, P<0.05; r s:kuru/kız=-0.58, P<0.05) and after (r s:sulu/kuru=-0.86, P<0.001; r s:sulu/kız=0.69, P<0.05; r s:kuru/kız=-0.64, P<0.05) opening to tourism were all significant. The correlations between the Sulu/Kuru and the Kuru/Kız Caves were negative but positive between the Sulu/Kız Caves. The directions of these correlations were the same before and after opening for tourism.
Table 2 The mean numbers of bats in the winter (November–March) and summer (April–October) in the three parts of the Dupnisa Cave System (Fig. 1), and statistical comparison with an independent samples t-test.
Discussion
A comparison of our results with previous studies (Albayrak, Reference Albayrak1993; Benda & Horacek, Reference Benda and Horacek1998; Furman & Özgül, Reference Furman and Özgül2004; Paksuz, Reference Paksuz2004; Paksuz et al., Reference Paksuz, Özkan and Postawa2007) shows that a total of 17 species of bats have been recorded in the Dupnisa Cave System. The two species that we did not record are Barbastella barbastellus and Plecotus austriacus (Benda & Horacek, Reference Benda and Horacek1998; Furman & Özgül, Reference Furman and Özgül2004). These 17 species comprise 45% of the 38 bat species known from Turkey and therefore this cave system is of national importance for bat conservation. Five of the species are categorized on the IUCN Red List (IUCN, 2010): R. mehelyi and M. capaccinii as Vulnerable, and R. euryale, M. bechsteinii and M. schreibersii as Near Threatened.
The protection of roosting sites is an essential component of any strategy for the conservation of bats. A study of the effect of cave tours on a maternity colony of bats found them to be negatively affected by light and noise (Mann et al., Reference Mann, Steidl and Dalton2002). Illumination located in caves for a long time causes changes in the formation of a cave and in its ecosystem. In Greece (Paragamian et al., Reference Paragamian, Nikoloudakis, Papadatou and Sfakianaki2004; Papadatou et al., Reference Papadatou, Butlin, Pradel and Altringham2009), Bulgaria (Beshkov, Reference Beshkov and Meine1998) and Turkey (Özgül et al., Reference Özgül, Bılgın, Furman and Woloszyn2000; Furman & Özgül, Reference Furman and Özgül2002, Reference Furman and Özgül2004; Albayrak, Reference Albayrak and Albayrak2005) suitable underground areas have deteriorated and cave-dwelling bat populations have declined because of such effects. Many caves in Turkey and Bulgaria (Petrov, Reference Petrov2008) that were opened to tourism were closed with gates that did not allow bats to fly in or out, and in Turkey visitor schedules for caves opened for tourism have generally not taken into account the seasonal use of the caves by bats.
Based on the long-term monitoring data that we collected in the Dupnisa Cave System the visitor schedule was designed to take into account the seasonal use of the caves by bats, to minimize disturbance. Sulu Cave is closed to tourism between 15 November and 15 May because the cave is used by hibernating bats during these dates. Kuru Cave is open to visits during the whole year because roosting colonies are located away from the tourist circuit. Kız Cave is closed to tourism because it is used for hibernating and breeding/nursing throughout the year. The entrances to the cave system for tourists were closed with horizontal angle iron gates to control human disturbance. Additional precautions were taken to protect the bats and the cave system: the time of visits has to be an hour after sunrise and an hour before sunset because of bat activity, visits are controlled by security guards all year and are only conducted in groups (with time limits and only on set routes), illumination inside the cave system is turned off when there are not any visitors and when it is not visiting time, and information notes about the bats and the cave system have been placed in suitable places at the entrances of the caves.
The distribution of bat species is significantly correlated with the type and size of cave. Large natural caves deserve special consideration for protection because of their high species richness and abundance (Niu et al., Reference Niu, Wang, Zhoa and Liu2007). The three caves of the Dupnisa Cave System have a variety of microclimates and topography (Paksuz et al., Reference Paksuz, Özkan and Postawa2007) and consequently host large, multi-species bat assemblages. During foraging and swarming bats fly in and out of a cave repeatedly, night after night (Thomas et al., Reference Thomas, Fenton and Barclay1979; Parsons et al., Reference Parsons, Jones, Davidson-Watts and Greenaway2003; Rivers et al., Reference Rivers, Butlin and Altringham2006) and spacing of bars in any gate is critical. The recommended minimal horizontal bar spacing is 150 mm (Tuttle, Reference Tuttle, Aley and Rhodes1977; Tuttle & Taylor, Reference Tuttle and Taylor1998; Burghardt, Reference Burghardt, Vories and Throgmortan2000; Powers, Reference Powers, Vories, Throgmortan and Harrington2002; Martin et al., Reference Martın, Leslıe, Payton, Puckette and Hensley2003; Pugh & Altringham, Reference Pugh and Altringham2005; Slade & Law, Reference Slade and Law2008). The bars in the two gates of the Dupnisa Cave System have gaps of 200 mm because of the large numbers of bats in the cave system. Ludlow & Gore (Reference Ludlow and Gore2000) demonstrated that, when given a choice, bats use ungated entrances more than gated ones. The other entrances of the cave system, which are located away from the tourist circuits, were therefore left ungated to minimize the negative effects of the two gates. Gated cave entrances prevent human disturbance and can increase the protection of bat populations but their effects on bats have not been adequately assessed (Tuttle, Reference Tuttle, Aley and Rhodes1977; Humphrey, Reference Humphrey1978; Tuttle & Stevenson, Reference Tuttle, Stevenson, Zuber, Chester, Gilbert and Rhodes1978; Richter et al., Reference Richter, Humphrey, Cope and Brack1993; Martin et al., Reference Martın, Leslıe, Payton, Puckette and Hensley2003).
In the Dupnisa Cave System the highest total number of bats previously recorded in a single survey was 33,000, in the winter of 2001 (Furman & Özgül, Reference Furman and Özgül2004) whereas in our study we recorded 42,800 individuals in January 2003 and 54,600 individuals in January 2004 (Table 1). The total number of bats in the cave system significantly increased after opening for tourism (2004–2008) compared to before (2002–2003). This increase was significant only in Kız Cave, which is closed to tourism and ungated. This increase may indicate that the bats prefer to use ungated entrances, as found by Ludlow & Gore (Reference Ludlow and Gore2000).
Before the Dupnisa Cave System was open to tourism it had been visited frequently by cavers and treasure hunters. These uncontrolled visits probably disturbed the bats and the increase in the total number of bats following the opening for tourism may be related to the control of visits by the installation of gates and the visitor schedule.
The three parts of the Dupnisa Cave System were used by bats to varying degrees in winter and summer. Sulu Cave and Kız Cave were used mainly by hibernating bats whereas Kuru Cave was mainly used as a nursery roost. The negative correlations between the Sulu/Kuru and the Kuru/Kız Caves indicated that when the number of bats in one cave increased it decreased in the other cave because of movements between the caves. However, the positive correlation between the Sulu/Kız Caves indicated that the numbers of bats in the caves increased and decreased in the same seasons. As these correlations in numbers of bats between the different parts of the cave system were preserved following gating and opening for tourism it indicates that the seasonal use of the cave system by bats were not affected by controlled visitation.
The results of our surveys of the Dupnisa Cave System show that gating of entrances and visits by tourists are not necessarily incompatible with the use of caves by bats for both hibernating and nursing. Understanding how the three caves are used seasonally by the bat community, and for what purposes (hibernation vs nursing), was critical for the establishment of an appropriate management plan for tourism.
The density and species richness of cave-dwelling bat communities depend on the presence of suitable types of caves and their microclimates, and roosting sites are limited and are therefore important for bat conservation. The Dupnisa Cave System may become more important for the cave-dwelling bats of southern Europe because the three caves have different microclimatic conditions and thus provide alternative microclimates in different seasons and caves. Studies need to be conducted on the behaviour of bats within this cave system and on the system's microclimate, which could potentially be modified by tourism activities, and the present monitoring needs to continue.
Acknowledgements
We thank Professor Cengiz Kurtonur and Dr Ebru Buruldağ for their help with this study, and Muharrem Obuz for accommodation and other assistance. This study was partially supported by the Kırklareli Governorship.
Biographical sketches
Serbülent Paksuz studies the population and conservation ecology of bats. He specializes in cave-dwelling bats and carries out research on seasonal population dynamics and roost selection. He is currently studying the impacts of human activities on bat colonies in caves open to tourism and is developing management plans for protection of bat communities in caves. He is also interested in the species diversity and distribution of mammals in Turkey. Beytullah Özkan has conducted ecological and taxonomic studies on mammals for more than 25 years and specializes in the taxonomy of rodents. He also leads biodiversity projects and fauna surveys and his current concerns are for bats and their conservation.






