Introduction
Increasing human populations and conversion of forest to agricultural land mostly have a negative impact on wildlife by reducing and isolating habitat and ranging areas and increasing geographical and ecological overlap between humans and wildlife (Woodroffe et al., Reference Woodroffe, Thirgood and Rabinowitz2005). The nature of human–wildlife interactions varies but is often characterized by increased resource competition and conflict, for example over crops (Paterson & Wallis, Reference Paterson and Wallis2005). Human–wildlife conflict is an important issue because it compromises conservation initiatives and threatens the economic and social security of rural people (Hill et al., Reference Hill, Osborn and Plumptre2002). A cross-disciplinary approach to this issue could facilitate our understanding of the realities facing humans and wildlife and the sustainability of their relationships in anthropogenic habitats.
Human perceptions of crop raiding by wildlife differ according to cultural attitudes and practices (Treves, Reference Treves, Manfredo, Vaske, Brown and Decker2008). In general people seem less tolerant of wildlife raiding cash crops (i.e. those that farmers rely on for an income) and important subsistence crops than domestic crops (Hill et al., Reference Hill, Osborn and Plumptre2002). This is probably influenced by people's capacity to absorb the costs of crop raiding and is linked to aspects of development, including various socio-economic factors such as reliance on a cash income from crop sales (Dickman, Reference Dickman2010). The way in which different species exploit a crop may also influence people's tolerance of raiding behaviour. For example, raiding by baboons (Papio spp.) in Uganda is rarely tolerated as they are considered destructive and wasteful compared to other species, including chimpanzees Pan troglodytes schweinfurthii (Hill & Webber, Reference Hill and Webber2010). In the rare instances in which crop raiding by wildlife provides benefits to farmers, negative perceptions can persist: for example, raiding of coconut Cocos nucifera by red colobus monkeys Procolobus kirkii in Zanzibar probably has a pruning effect that increases coconut productivity yet farmers maintain that this species damages harvests (Siex & Struhsaker, Reference Siex and Struhsaker1999). The expectation of compensation may also influence attitudes towards crop raiding (Nyhus et al., Reference Nyhus, Osofsky, Ferraro, Madden, Fischer, Woodroffe, Thirgood and Rabinowitz2005). Large-bodied animals such as elephants (Loxodonta spp.) are often less tolerated than smaller animals because of real or perceived threats to human safety (Hoare, Reference Hoare1999).
Non-preferred crop types can be used to reduce crop damage by wildlife, for example through the establishment of buffer zones of unpalatable crops at forest edges and modification of the principal crops grown (Hockings & Humle, Reference Hockings and Humle2009). Such conflict mitigation techniques can aid wildlife conservation if they reduce the occurrence of retaliatory killings of so-called problem animals (Macfie, Reference Macfie2000). Parker & Osborn (Reference Parker and Osborn2006) noted two key properties regarding the suitability of such crops: firstly, they should be unpalatable to crop-raiding animals and, secondly, they should be economically valuable to the farmer. After testing the palatability of chilli (Capsicum spp.) to mammalian pests, including baboons, they suggested that chilli is less vulnerable to wildlife damage than other crops and is also an economically viable alternative for farmers in Zimbabwe. Similarly, tea Camellia sinensis plantations at the forest edge in Kibale National Park in Uganda are profitable to farmers, unattractive to crop-raiding animals, including chimpanzees, and seem to act as successful low-conflict barriers between wildlife and local people (Southworth et al., Reference Southworth, Hartter, Binford, Goldman, Chapman and Chapman2010).
Chimpanzees were declared extinct in Guinea-Bissau in 1988 (Lee et al., Reference Lee, Thornback and Bennett1988) but recent evidence indicates that a population of 600–1,000 remains, most in the coastal forests of Cantanhez National Park (Gippoliti et al., Reference Gippoliti, Embalo, Sousa, Kormos and Boesch2003). Suitable forest habitats in the Cantanhez region (extending beyond the Cantanhez Peninsula to include parts of the Cacine and Catio regions) decreased by c. 11% (270 km2 of a total study area of 2,723 km2) from 1986 to 2003 (Torres et al., Reference Torres, Brito, Vasconcelos, Catarino, Gonçalves and Honrado2010) and, depending on three scenarios of chimpanzee density, will have resulted in a decrease of 157–1,103 chimpanzees. For the low-density, or worst-case, scenario (0.5 individuals km−2) the population of chimpanzees in the Cantanhez region is predicted to be < 400 individuals. However, using questionnaire data collected from local hunters, Brugiere et al. (Reference Brugiere, Badjinca, Silva and Serra2009) suggested that chimpanzees were present around all villages surveyed between the Corubal river and the border with Guinea (n=70) in southern Guinea-Bissau. Chimpanzees in this region continue to be severely threatened by habitat isolation and increasing anthropogenic activities, primarily agricultural expansion. Unless resource conflict levels with humans are understood and mitigation strategies implemented, chimpanzees are likely to become extinct in Guinea-Bissau (Casanova & Sousa, Reference Casanova and Sousa2007; Brugiere et al., Reference Brugiere, Badjinca, Silva and Serra2009).
Despite their importance, data on the role of particular crop types and parts, especially cash crops, in determining human–wildlife conflict are scarce. Here we present data on the co-utilization of a nationally important cash crop, cashew Anacardium occidentalis, by people and a chimpanzee Pan troglodytes verus community inhabiting a forest–agricultural matrix in central-southern Cantanhez National Park in Guinea-Bissau.
Study area
The 13,948 km2 Republic of Guinea-Bissau lies on Africa's north-western coast. Cantanhez National Park is in the south-west, in the Tombali Administrative Region (Fig. 1). Scattered within the 1,057 km2 Park are c. 110 villages, with a population density of c. 20 people km−2 (Temudo, Reference Temudo2009). Several primate species occur in the Park (Gippoliti & Dell'Omo, Reference Gippoliti and Dell'Omo1996): the western chimpanzee, colobus monkeys (Procolobus badius temmincki and Colobus polykomos), Guinea baboon Papio papio, grivet monkey Cercopithecus aethiops sabaeus, Campbell's monkey Cercopithecus campbelli and Senegalese galago Galago senegalensis.
Fig. 1 The location of Cantanhez National Park (shaded) in the Tombali Administrative Region in south-west Guinea-Bissau, West Africa. The black square indicates the location of the study site (Fig. 2).
Cantanhez National Park is a mosaic of forest, savannah, mangroves and agricultural areas (Gippoliti et al., Reference Gippoliti, Embalo, Sousa, Kormos and Boesch2003; Sousa et al., Reference Sousa, Barata, Sousa, Casanova and Vicente2011). Forests in the Park are classified as protected but are afforded little formal protection, with increasing clearance for subsistence cultivation and conversion into cashew plantations. Within the Park c. 4% of the forest is converted to cashew plantations annually (Barry et al., Reference Barry, Creppy, Wodon, Barry, Creppy, Gacitua-Mario and Wodon2007). Cashew farms cover 73% of the country's arable land and most farmers depend on the crop for cash income to buy imported rice (Barry et al., Reference Barry, Creppy, Wodon, Barry, Creppy, Gacitua-Mario and Wodon2007). Cashew is a preferred crop as it is drought resistant, produces fruit quickly after planting and is easily maintained.
A diversity of ethnic groups inhabit Cantanhez National Park. Cultural attitudes towards flora and fauna held by some groups, including the Nalu, offer a degree of traditional habitat protection, with certain forests and tree species having symbolic and religious meanings (Frazão-Moreira, Reference Frazão-Moreira2001). Local taboos prohibit hunting of chimpanzees for meat, as they are considered too similar to humans (Costa et al., Reference Costa, Frias, Casanova and Sousa2008).
Methods
We collected data during the dry season between February and May 2009, coinciding with the cashew fruiting period, in the vicinity of the villages Caiquene and Cadique-Nalu (hereafter Cadique). Both are small villages (6,602 and 28,485 m2 respectively), with a combined human population of c. 400 predominantly of Nalu, but also Balanta, ethnicities. One chimpanzee community (unhabituated to researchers) populates the forest–farm matrix around these villages and has a home range area of c. 10 km2 (latitude 11° 12′–11° 15′ N and longitude 15° 04′–15° 06′ W). This Caiquene–Cadique community comprises a minimum of 31 chimpanzees, including at least 14 adult males and 11 adult females (KH, unpubl. data). There are no detailed behavioural and ecological data for this chimpanzee community but individuals are frequently observed crossing roads and show little fear of people (KH, unpubl. data). Chimpanzee communities often exhibit specific feeding and behavioural adaptations to habitats, including anthropogenic areas (McLennan, Reference McLennan2008; Hockings et al., Reference Hockings, Anderson and Matsuzawa2009). Based on behavioural observations (sightings, vocalizations, road-crossing points) of chimpanzees, the location of chimpanzee sign (nests, faeces, knuckle prints, feeding remains), local reports, natural and man-made barriers and preliminary genetic analyses, chimpanzees at this site are believed to belong to one community (KH, unpubl. data; Rui Sá, unpubl. data). At the time of this study there had been no reports of attacks by chimpanzees on local people at this site. This ensured that other chimpanzee behaviours were not influencing people's perceptions of cashew consumption and potential conflict with chimpanzees.
We mapped the cashew fields (n=26) within the known home range of the Caiquene–Cadique chimpanzee community using a global positioning system and produced maps with ArcView v. 9.3 (ESRI, Redlands, USA). We systematically monitored cashew fields bordering the main forest block (i.e. those considered particularly accessible to this community of chimpanzees; n=17) on a weekly basis from the beginning of March (when the cashew apple or pseudofruit, hereafter the fruit, started ripening) until the end of May (when fruit production ended), recording the presence/absence of fresh traces of raiding by chimpanzees, i.e. cashew fruit wadges. The production of compact wadges during the consumption of certain fruits is typical of the feeding behaviour of chimpanzees (Nishida et al., Reference Nishida, Wrangham, Goodall and Uehara1983). The seedless cashew wadges are discarded once the chimpanzee has extracted the juices and are easily distinguishable from the cashew feeding traces left by other non-human primates. For identification of locations of raids and to supplement data obtained during monitoring of cashew fields we attempted to observe chimpanzees raiding in cashew fields, and areas were also monitored for wadges following local reports of raiding.
During the mapping stage we informally asked the owners of fields and orchards if chimpanzees visited their grounds and, if so, whether the chimpanzees consumed cashews and approximately how often they visited. If farmers reported that chimpanzees consumed cashews perceptions towards raiding were recorded, when possible, in informal interviews (nine out of 12 farmers). Farmers were not prompted and were free to discuss any aspect of raiding by chimpanzees (Bernard, Reference Bernard2002). Direct questioning about farmers' perceptions was avoided so as not to inflate any potential conflict situations (Hockings & Humle, Reference Hockings and Humle2009). Anthropological protocols followed the ethical guidelines proposed by the Association of Social Anthropologists of the UK and Commonwealth.
Results
Within the known home range of the Caiquene–Cadique chimpanzee community there are 31 cultivated areas (discrete non-overlapping areas separate from other cultivated areas), including trees, orchards and fields, which total 423,084 m2 (mean 14,589±SE 6,828 m2, range 472–200,000 m2). Rice paddy fields were excluded from our assessments. Eighteen crop foods are cultivated by local people including papaya Carica papaya, cashew, cowpea bean Vigna unguiculata, mango Mangifera indica and orange Citrus sinensis, and are mostly located in small patches proximal to houses. Of the cultivated areas, 92% contain cashew, totalling 388,889 m2 (mean 15,555±SE 7,868 m2), and cashew plantations comprise 3.9% of the chimpanzees' known home range.
Direct observations and identified traces confirmed that chimpanzees raided cashew fruit in eight of the 17 monitored locations. Farmers reported that cashew is raided in a further four locations, although these could not be confirmed through trace identification (Fig. 2). Five of the 12 areas raided border settlements and all are located within 1 km of settlements and roads. Cashew raiding remains unconfirmed in 11 of 26 mapped cashew locations as traces were not found and/or we were unable to obtain information from owners of fields/orchards. Farmers reported that chimpanzees never visit three areas where cashews are cultivated on the easterly and westerly outskirts of their home range.
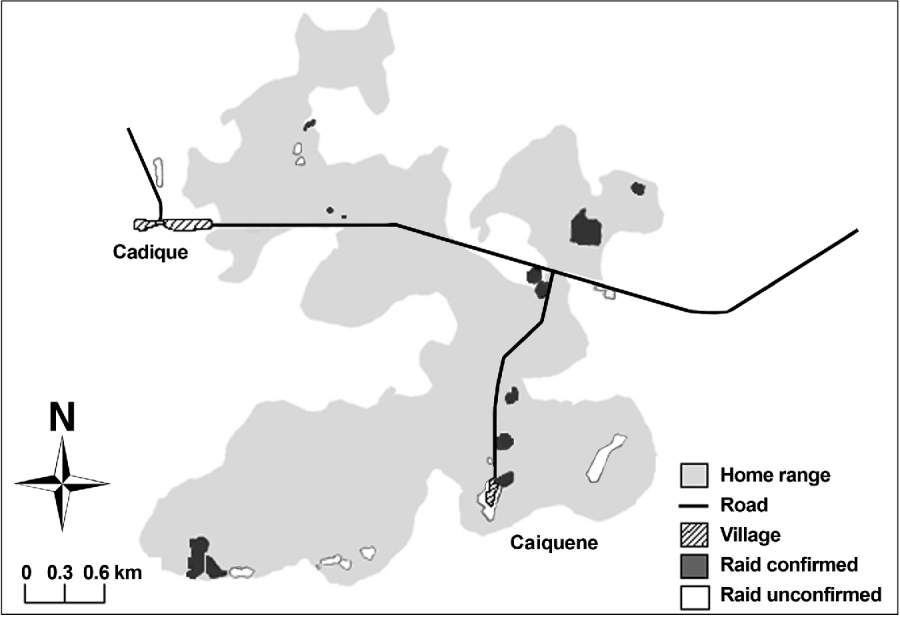
Fig. 2 The known home range of the Caiquene–Cadique chimpanzee Pan troglodytes verus community in the vicinities of the villages of Cadique and Caiquene. This area is a mixture of primary and secondary forest, mangrove, savannah and cultivated and fallow fields. Cashew fields are highlighted and categorized according to whether raiding was confirmed (through direct observations, traces or local reports) or unconfirmed.
Eight of the nine farmers interviewed estimated that chimpanzees visited their cashew fields at least once per week. All of the farmers interviewed reported that the cashew seed (more commonly referred to as the nut) is never consumed by chimpanzees. The nut is surrounded by a double shell containing anacardic acid, which is a skin irritant and must be removed before the nut is processed. Our direct observations (n=5 in total, involving 2–11 individuals) confirmed that chimpanzees take unspoilt fruits from the ground (ripe fruits drop to the ground where they rot quickly) and from the trees. Chimpanzees bite off the fruit, discard the nut, place several fruits in the mouth and make a wadge; the discarded wadges are easily identifiable (Plate 1a). Two of the nine farmers interviewed mentioned that chimpanzees sometimes damage cashew trees by snapping branches to obtain fruits but most, eight of the nine farmers, reported that, after eating the fruits, chimpanzees leave the nuts in piles thereby making nut collection easier for the farmer. We never observed such piles while monitoring fields.
Cashew fruit is fragile and ferments quickly, making it unsuitable for transport and retail. For this reason the farmers reported that the fruits are not sold but are normally discarded after removal of the nut (Plate 1b). However, the Balanta ethnic group commonly make an alcoholic beverage from the fruit; the Nalu, who are predominantly Muslim, abstain. In Balanta areas two of the four farmers interviewed reported that raiding by chimpanzees is tolerable when fruits are plentiful but not during periods of low availability when the fruits are required to make cashew liquor.

Plate 1 (a) Feeding remains of cashew fruit with nut attached (top) and a chimpanzee cashew fruit wadge (bottom). (b) Rotten cashew fruits are cleaned from the orchard floor by farmers, often resulting in mounds of decomposing cashew fruits.
Discussion
Parker & Osborn (Reference Parker and Osborn2006) proposed that low conflict crops should be economically valuable to farmers and unpalatable to crop-raiding animals. Perhaps unusually for a cash crop our data show that humans and wildlife can exploit the same cultivated resource with minimal competition and conflict if separate parts of the crop are consumed and farmers perceive some advantages to raiding. In the case of cashew, humans mostly use the marketable nut and chimpanzees only consume the fruit, and most farmers described the benefits of chimpanzees placing cashew nuts in manageable piles for people to harvest. More systematic observations of chimpanzees are required to support this belief, as humans also leave behind the detached nut after consuming the fruit. However, some Balanta farmers expressed their irritation when fruits were raided during periods of low availability because of their use in the production of local liquor. These observations highlight how different cultural practices can affect levels of human–wildlife resource competition and conflict mitigation measures.
Chimpanzees exploited many cashew fields throughout their home range but cashew-raiding rates could not be calculated for several reasons: (1) the chimpanzees are unhabituated and therefore behavioural observations of crop raids were opportunistic, (2) chimpanzees extract the juices from the cashew fruit and these cannot be identified in faeces, (3) damage to the cashew tree resulting from raiding (e.g. broken branches) is not predictable and therefore traces are not an independent measure of raiding rate, (4) chimpanzees often transport cashew fruits and wadges from fields into the forest (KH, pers. obs.) making accurate quantification of remains difficult, and (5) discarded wadges decompose rapidly. This emphasizes the need for a standardization of data collection methods in human–wildlife conflict research to facilitate meaningful comparisons of resource competition between different crop types and parts and geographical areas. Because of the methods used in this study the absence of chimpanzee traces within a cashew field did not confirm the absence of raiding behaviour. As a result, the geographical and anthropogenic characteristics of raided cashew fields, such as proximity to other attractive cultivated areas and human presence, were not examined in detail. The value of a cross-disciplinary approach such as that adopted in this study (i.e. combining systematic monitoring of crop fields or wildlife behaviour with interviews or focus groups with local people) is becoming increasingly acknowledged and we suggest that it should be extended to other sites where chimpanzees eat cashew fruits, to test the wider applicability of our findings. The cashew fruit is notably high in calcium, iron and vitamins C and B1; it would be of value to examine the nutritional and seasonal importance of this fruit to chimpanzees, whether fruits are raided in response to shortages of wild food or whether they are taken in preference to lower-quality wild foods (Naughton-Treves et al., Reference Naughton-Treves, Treves, Chapman and Wrangham1998; Hockings et al., Reference Hockings, Anderson and Matsuzawa2009).
Although we found that raiding of cashews currently causes little direct conflict between farmers and chimpanzees, severe problems are associated with land clearing for cashew production, principally that high levels of deforestation, desertification and forest fragmentation threaten chimpanzee habitat, and these processes will ultimately increase conflict levels. If land clearing continues, it is probable that chimpanzees will forage increasingly on crops, including cashew, because the natural food supply may be insufficient to support them (see Campbell-Smith et al., Reference Campbell-Smith, Campbell-Smith, Singleton and Linkie2011, for an example concerning the orang-utan Pongo abelli). This will also bring chimpanzees into closer contact with people, increasing the likelihood of additional problems such as disease transmission and attacks on people (Woodroffe et al., Reference Woodroffe, Thirgood and Rabinowitz2005; McLennan, Reference McLennan2008; Hockings et al., Reference Hockings, Yamakoshi, Kabasawa and Matsuzawa2010). For these reasons it would be inadvisable to develop cashew plantations elsewhere in Africa on the basis that cashew is a low-conflict crop. A wild population of capuchin monkeys Cebus libidinosus in Fazenda Boa Vista, Brazil, frequently use tools to crack open the cashew shell to obtain the nut inside (Visalberghi & Sirianni, in press). As wild chimpanzees are prolific tool users, cracking open the shells of cashew is within this species' capabilities (Matsuzawa et al., Reference Matsuzawa, Humle and Sugiyama2011).
Reports from local farmers suggest that the raiding behaviours of chimpanzees on other crops, especially oranges, are less tolerated and cause more resentment. Any future land-use management schemes for Cantanhez National Park should advise against the establishment of plantations of potentially high-conflict crops within the Park and other protected areas, especially those that are not important human subsistence crops (Hockings & McLennan, Reference Hockings and McLennan2012). Attempts should be made to preserve key forests and ensure that connecting areas do not become impassable (i.e. through establishment of further cashew plantations) so that chimpanzee communities do not become more isolated, especially if human populations continue to increase within Cantanhez National Park.
Guinea-Bissau is the sixth largest exporter of unprocessed cashew nuts and local farmers rely heavily on an income from cashew, which is vulnerable to external markets. For example, cashew nut output in Guinea-Bissau declined by c. 30% in 1998 and global cashew prices dropped by > 50% in 2000 (Barry et al., Reference Barry, Creppy, Wodon, Barry, Creppy, Gacitua-Mario and Wodon2007). When human incomes are unpredictable people may be less tolerant towards any form of resource competition with wildlife and fall back on alternative cultivated resources, possibly higher-conflict cash crops. Conserving biodiversity in anthropogenic habitats requires integrating sustainable resource use and conflict mitigation strategies with the protection of core conservation areas. The imperative to conserve populations on the brink of extinction demands the coexistence of people and threatened wildlife and, by ensuring that conflict levels over crops (especially those with high economic importance) remain low, the costs of living in proximity to wildlife can be reduced.
Acknowledgements
We thank the Instituto da Biodiversidade e das Áreas Protegidas, Guinea-Bissau. We also thank our local assistants, in particular M. Cassama and D. Indjai, for their invaluable help and the villagers for continuing support. We are grateful to C. Casanova and M. McLennan for helpful comments. This work was supported by a post-doctoral research grant (SFRH/BPD/38595/2007) to KH and a research grant (PPCDT/ANT/57434/2004) to CS from Fundação para a Ciência e a Tecnologia, Portugal, and fieldwork expenses for KH from Conservation International.
Biographical sketches
Kimberley J. Hockings and Claudia Sousa conduct cross-disciplinary research at the interface of conservation biology, animal behavioural ecology and social anthropology. Recognizing a growing need to conserve wildlife that conflicts with people, Kimberley has conducted research on the ecological drivers of resource competition and conflicts between humans and chimpanzees, and on technical measures to resolve such conflicts. In 2003 Claudia co-established a research site in Guinea-Bissau, Project Dari (‘Dari’ meaning chimpanzee in the local Creole), to reduce biodiversity loss and help preserve important wildlife habitat. Both authors have a commitment to using science to influence policy and conservation action, especially for primates in Guinea and Guinea-Bissau.





