Introduction
Knowledge of prey selection and diet is important for understanding the life history strategies of carnivores and for planning the conservation of an apex predator (Miquelle et al., Reference Miquelle, Smirnov, Quigley, Hornocker, Nikolaev and Matyshkin1996). The density of carnivores is related to habitat quality, in particular to the availability of prey (Fuller & Sievert, Reference Fuller, Sievert, Gittleman, Funk, Macdonald and Wayne2001; Carbone & Gittleman, Reference Carbone and Gittleman2002; Andheria et al., Reference Andheria, Karanth and Kumar2007; Karanth & Nichols, Reference Karanth, Nichols, Tilsonand and Nyhus2010). To enhance the cost–benefit ratio of energetic intake, large carnivores may visit areas close to, or even inside, human settlements (Gehrt et al., Reference Gehrt, Riley and Cypher2010; Yirga et al., Reference Yirga, DeIongh, Leirs, Gebrihiwot, Deckers and Bauer2012; Athreya et al., Reference Athreya, Odden, Linnell, Krishnaswamy and Karanth2013). Fear of carnivores, especially in the context of livestock depredation, can negatively affect people's well-being (Inskip & Zimmermann, Reference Inskip and Zimmermann2009). It has been shown that the abundance and availability of wild and domestic prey is the predominant factor that determines the potential carrying capacity of human-dominated landscapes for large carnivores (Boitani & Powell, Reference Boitani and Powell2012). In human-dominated landscapes in Brazil, Nepal and Kenya (Schaller, Reference Schaller1983; Seidensticker et al., Reference Seidensticker, Sunquist, McDougal, Daniel and Serrao1990; Mizutani, Reference Mizutani1999) the biomass of potential domestic prey of carnivores was higher than that of wild prey species. Discarded food waste and pet food may also contribute to the food resources of carnivores (Gehrt et al., Reference Gehrt, Riley and Cypher2010). This reduces carnivores’ fear of humans and, in consequence, the density of carnivores in urban or semi-urban areas can be higher than in the wild (Butler et al., Reference Butler, Linnell, Morrant, Athreya, Lexcureux, McKeown and Gompper2014).
In India, the intrusion of large predators into urban areas is well documented. Wolves Canis lupus (Jhala & Giles, Reference Jhala and Giles1991), Asiatic lions Panthera leo persica (Meena et al., Reference Meena, Jhala, Chellam and Pathak2011), striped hyaenas Hyaena hyaena (Singh et al., Reference Singh, Gopalaswamy and Karanth2010) and tigers Panthera tigris (Karanth & Gopal, Reference Karanth, Gopal, Woodroffe, Thirgood and Rabinowitz2005) are known to attack livestock and persist in human-dominated landscapes. The leopard Panthera pardus fusca also lives successfully in the proximity of people (Athreya et al., Reference Athreya, Thakur, Chaudhuri and Belsare2004). Its broad diet includes amphibians, arthropods and carrion, reducing dependence on water sources, which is obtained from the prey (Daniel, Reference Daniel2009; Kshettry et al., Reference Kshettry, Vaidyanathan and Athreya2018). Compared to larger felids, the small body size of the leopard reduces the territory required to sustain a population and allows it to survive and thrive in proximity to people (Daniel, Reference Daniel2009; Kshettry et al., Reference Kshettry, Vaidyanathan and Athreya2018).
To examine resource utilization by leopards in a rural, semi-urban human-dominated landscape, we analysed the diet of leopards of the Jhalana Reserve Forest. Because the Reserve is in close proximity to and surrounded by human habitations, leopards have little fear of people and often feed opportunistically on easily accessible domestic prey (Athreya et al., Reference Athreya, Odden, Linnell, Krishnaswamy and Karanth2013, Reference Athreya, Odden, Linnell, Krishnaswamy and Karanth2014). We thus hypothesized that the leopards' diet comprises principally of domestic animals. Our objectives were to identify the leopards' key prey species and examine their importance for human–leopard coexistence and acceptance of the presence of leopards in the urban landscape (Kumbhojkar et al., Reference Kumbhojkar, Yosef, Benedetti and Morelli2019).
Study area
The 29 km2 Jhalana Reserve Forest lies on the south-east border of the city of Jaipur in north-west India (Fig. 1). It was designated on 21 November 1961 in accordance with the provisions of Rajasthan Forest Act 1953. The Reserve has a mean altitude of 516 m, with higher elevations in the north in the form of low, flat-topped hills, and is characterized by tropical dry deciduous forest. During the 1980s the main valley was planted with the native species Acacia tortilis and Acacia senegal.
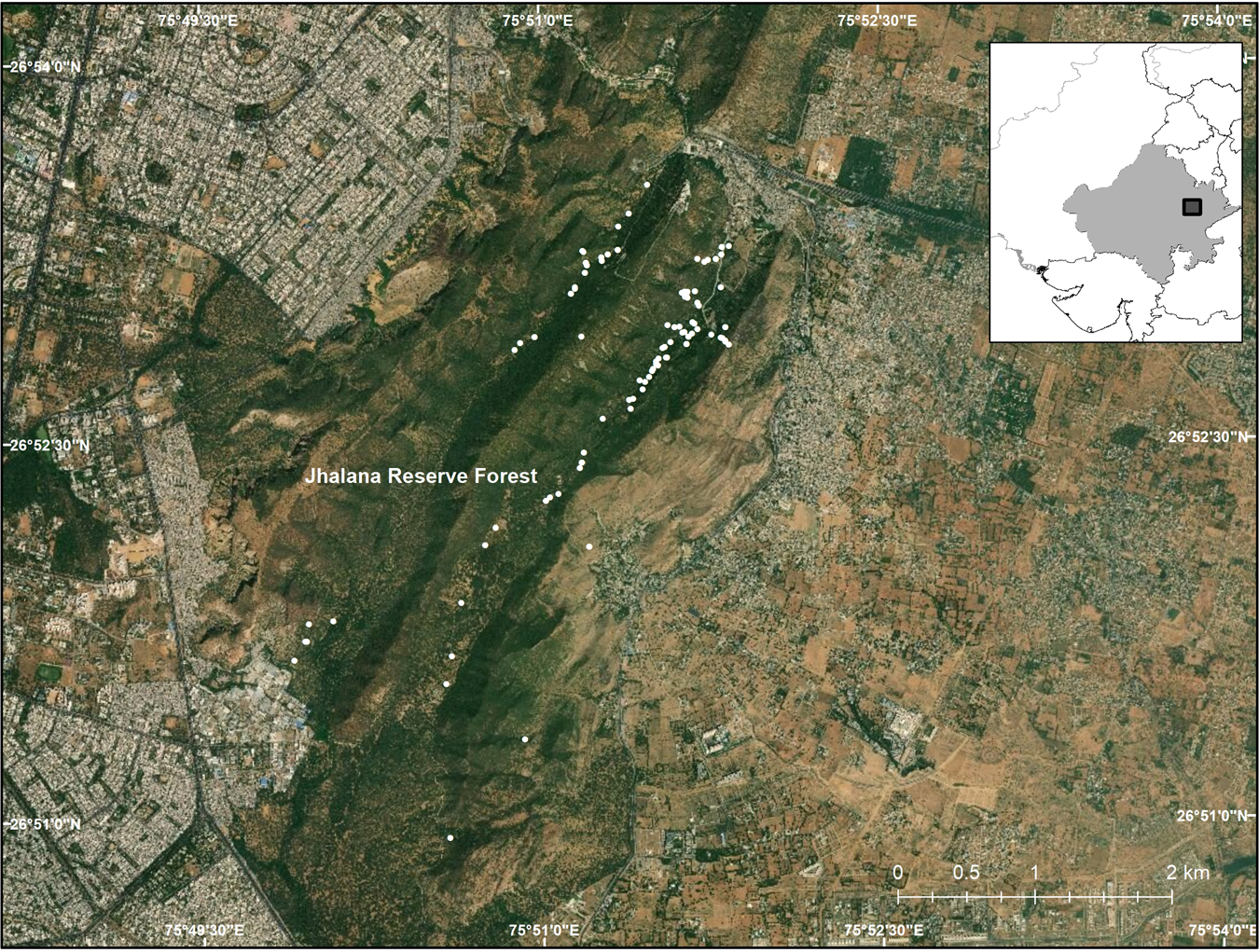
Fig. 1 Jhalana Reserve Forest in north-west India, and the surrounding city of Jaipur, with the locations of the leopard Panthera pardus fusca scats collected.
Methods
The population of leopards in the Reserve has been estimated, using camera traps and recognition of individuals, to comprise 25 individuals (authors, unpubl. data). In a 2017 survey of the Reserve we found that leopards used trails and tourist routes for defecation. We monitored the trails, collecting 138 scat samples in the dry season of November 2017–April 2018 (Kumbhojkar et al., Reference Kumbhojkar, Yosef, Benedetti and Morelli2019; Fig. 1).
The identity of leopard scats was confirmed using their occurrence in scrapes characteristic of those made by large felids (the leopard is the only large felid present in the Reserve). Trained volunteers wearing gloves used forceps to collect the scats. A small portion of each scat was left, so as to not disrupt the natural markings of the leopards (Schwarz & Fischer, Reference Schwarz and Fischer2006). The location of each scat was noted, with a GPS, at the time of collection. Scats were stored in numbered polythene, zip-lock bags. Preliminary observations such as the presence of bones, claws, skin and other biological remains were noted if appropriate.
Highly degraded scats (n = 6; 4.3%) were excluded from the analysis and only well-preserved scats (n = 132) were analysed. They were washed under running water and sundried. The cuticular and medullar patterns of any hair remains were observed and photographed under a compound microscope. Scat analysis was based on Mukherjee et al. (Reference Mukherjee, Goyal and Chellam1994), Mukherjee & Mishra (Reference Mukherjee and Mishra2001), diet-related studies (Karanth & Sunquist, Reference Karanth and Sunquist1995; Biswas & Sankar, Reference Biswas and Sankar2002; Sankar & Johnsingh, Reference Sankar and Johnsingh2002; Andheria et al., Reference Andheria, Karanth and Kumar2007; Khorozyan et al., Reference Khorozyan, Malkhasyan and Abramov2008; Odden & Wegge, Reference Odden and Wegge2009), and our own collections of prey remains. All hair, hooves, claws, teeth, nails and bones were separated for further analysis. Prey species were identified based on comparison with reference slides of hair samples from domestic animals in the study area and from reference slides of hair samples from wild prey and from previous studies (Oli, Reference Oli1993; Tiwari, Reference Tiwari2008).
To assess if the sample size was sufficient for accurate diet analysis (Edgaonkar & Chellam, Reference Edgaonkar and Chellam1998; Kshettry et al., Reference Kshettry, Vaidyanathan and Athreya2018) we applied the rarefaction method, implemented in EstimateS 9.1 (Colwell, Reference Colwell2005). This method estimates the expected cumulative number of species, with 95% confidence intervals (the Mao Tau estimator; Colwell, Reference Colwell2005).
We calculated the relative biomass of prey species consumed using the regression equation Y = 0.35X + 1.98Y, where Y is the mass of prey consumed per scat and X is the mean mass of the prey (cf. Ramesh et al., Reference Ramesh, Snehalatha, Sankar and Qureshi2009). The regression equation is derived based on feeding trials with captive mountain lions Puma concolor (Ackerman et al., Reference Ackerman, Lindzey and Hemker1984) to calculate relative biomass based on their relative proportions in scats. As mountain lions and leopards are similar in size, this method has been used previously for leopards (Karanth & Sunquist, Reference Karanth and Sunquist1995; Andheria et al., Reference Andheria, Karanth and Kumar2007; Khorozyan et al., Reference Khorozyan, Malkhasyan and Abramov2008; Odden & Wegge, Reference Odden and Wegge2009; Wegge et al., Reference Wegge, Odden, Pokharel and Storaas2009; Athreya et al., Reference Athreya, Odden, Linnell, Krishnaswamy and Karanth2014).
The frequencies of occurrence of prey species (the per cent of the total number of scats that contained a specific prey item) were calculated but, as smaller prey species may contribute more to scats than larger species (Karanth & Sunquist, Reference Karanth and Sunquist1995; Klare et al., Reference Klare, Kamler and Macdonald2011), this variable may potentially be misleading. The relative biomass (D) was therefore calculated using:
where A is the frequency of occurrence of the prey species. The relative per cent of each prey species consumed (E) was obtained using:
 $$E = \displaystyle{{\displaystyle{D \over X}} \over {\sum {\displaystyle{D \over X}} }} \times 100$$
$$E = \displaystyle{{\displaystyle{D \over X}} \over {\sum {\displaystyle{D \over X}} }} \times 100$$Because we found that the correlations between Y, D, and E (R 2 0.28–0.99) were all significant at P < 0.01, we only tested differences in biomass (D) between domestic and wild prey species by t test with Cochran–Cox adjustment (Zar, Reference Zar1996). All calculations were conducted in R 3.6.0 (R Core Team, 2013).
Results
The 132 scats had a mean of 9.8 ± SE 0.24 (9.3–10.3 95% CI) prey species. Most of the scats (108; 82%) were found alongside trails or jeep tracks. Of the 2,640 hairs selected from scats, 158 (6%) were unidentified. The scats contained a total of 12 prey taxa: 11 individual species and rodents, which were not identified to species level (Fig. 2). Seventy-nine per cent (105) of the scats contained only one prey species; 21% contained two or more species. The individual-based rarefaction curve shows that the increase in the cumulative number of species in scats was rapid for the first c. 80 scats but began to level off thereafter (Fig. 3). In the 132 leopard scats the majority (89.5%) of prey frequency of occurrence comprised domestic animals (domestic dogs Canis lupus familiaris, 44%; domestic cats Felis catus, 13%; goats Capra aegagrus hircus, 16%; cattle Bos taurus, 15%) and wild prey comprised 11% of prey frequency of occurrence; the difference was significant (G test; G = 4.16, P < 0.001). The mean biomass of domestic animal species detected in the scats was 1.29 and that of wild prey was 0.02 kg; the difference was significant (t = 2.78, P = 0.038; Table 1).
Table 1 Mean weight, per cent frequency, mass consumed per scat, relative biomass consumed and relative per cent of prey, in decreasing order of frequency, for each of the 12 prey taxa recorded in the 132 scat samples of the leopard Panthera pardus fusca.

1From Karanth & Sunquist (Reference Karanth and Sunquist1995), Biswas & Sankar (Reference Biswas and Sankar2002), Sankar & Johnsingh (Reference Sankar and Johnsingh2002) and Andheria et al. (Reference Andheria, Karanth and Kumar2007).
2Y, mass of prey consumed per scat; D, relative biomass; E, relative per cent of each prey species consumed (see text for further details).

Fig. 3 Cumulative number of prey species in leopard scats (solid line), with 95% confidence interval (shaded area).

Plate 1 Leopard Panthera pardus fusca preying on a domestic dog that strayed into the boundaries of Jhalana Reserve Forest. Photo: Devam Shah.
Discussion
In urban landscapes the relationship between large felids and humans is complex, influenced by people's fear and awe (Boomgaard, Reference Boomgaard2001; Loveridge et al., Reference Loveridge, Wand, Frank, Seidensticker, Macdonald and Loveridge2010). Aversion occurs when the presence of a large felid results in damage to property or loss of human life (Treves et al., Reference Treves, Wallace, Naughton-Treves and Morales2006), with retaliatory killings resulting in significant felid mortality (Inskip & Zimmermann, Reference Inskip and Zimmermann2009). Studies have therefore focused on so-called human–felid conflict (Inskip & Zimmermann, Reference Inskip and Zimmermann2009). Dependence of felids on domestic livestock has rarely been observed, however; domestic livestock usually contribute only a small proportion of the biomass consumed by felids (Athreya et al., Reference Athreya, Odden, Linnell, Krishnaswamy and Karanth2014). In urban landscapes the biomass of domestic animals is usually high and usually exceeds that of wild prey in the neighbouring forests (Schaller, Reference Schaller1983). So, if domestic animals lack anti-predatory behaviour, they are more susceptible to attacks (Diamond, Reference Diamond2002). Jhalana Reserve Forest is surrounded by urban and rural areas with c. 4 million inhabitants (World Population Review, 2020). People living in the surrounding neighbourhoods and villages practice traditional livelihood professions such as livestock farming. Goats and the calves of cattle are available as leopard prey along with domestic dogs, cats and pigs. An estimated population of 36,580 domestic dogs was documented in a 2011 survey in Jaipur (Hiby et al., Reference Hiby, Reece, Wright, Jaisinghani, Singh and Hiby2011). This is a sizeable source of potential prey for the leopards. Hence, it is unsurprising that leopards move between the protected area and human habitat, despite the 5 m high barrier built around Jhalana to separate it from the city of Jaipur (Fig. 1). There are no data available on the densities or numbers of the leopards' wild prey species in Jhalana Reserve Forest. There are, however, data on the vaccination and sterilization of feral domestic dogs undertaken for the past 2 decades by the Help in Suffering Foundation, Jaipur (J. Reece, pers comm., 2019): a mean of c. 7,500 rabies inoculations were conducted annually over the past 21 years, to protect residents in the case of bites from feral dogs. Previous studies have shown that the availability of a large number of domestic animals in rural and urban areas facilitates the survival of leopards near human habitats (Athreya et al., Reference Athreya, Thakur, Chaudhuri and Belsare2004; Kshettry et al., Reference Kshettry, Vaidyanathan and Athreya2018). Persistence of leopard populations in human-dominated habitats beyond protected areas is dependent on a stable and abundant domestic prey base (Athreya et al., Reference Athreya, Odden, Linnell, Krishnaswamy and Karanth2013).
The Mao Tau diversity estimator indicated that 90–100 scat samples sufficed to give a representative sample of the species included in the diet of the leopards (Fig. 3). Biswas & Sankar (Reference Biswas and Sankar2002) considered that a minimum of 60 scats needed to be analysed to examine the prey base of tigers.
Scat analysis substantiated our hypothesis that the predominant prey of leopards in Jhalana Reserve Forest are domestic animals, presumably from the neighbouring city and villages. Our results corroborate the results of Daniel (Reference Daniel2009) and demonstrate that dogs are a major food resource for leopards in the Reserve (Plate 1). Scat analysis from the states of Maharashtra and Jammu and Kashmir have also reported the importance of dogs as prey for leopards (Edgaonkar & Chellam, Reference Edgaonkar and Chellam2002; Shah et al., Reference Shah, Jan, Bhat, Ahmad and Ahmad2009). In West Bengal, however, livestock (cattle and goats; 48%) predominated in the diet of leopards (Kshettry et al., Reference Kshettry, Vaidyanathan and Athreya2018), whereas cattle and goats comprised 31% of the diet in Jhalana Reserve Forest. In Ayubia National Park, Pakistan, the leopard similarly subsisted mainly on domestic animals, with goats predominating (64.9%) the frequency of occurrence (Shehzad et al., Reference Shehzad, Nawaz, Pompanon, Coissac, Riaz, Shah and Taberlet2015).
The differences between our results and other studies are a consequence of the high number of dogs and cats in our study area. The total dog and cat populations in the area are estimated to be c. 32,500 and 7,500 individuals, respectively (J. Reece, pers. comm., 2019); these species are thus widely available. Cattle and goats are relatively less abundant (31%) because these animals are herded during the day and protected in enclosures at night. Similarly, in Sanjay Gandhi National Park, Mumbai, leopards have adapted to the depleted wild ungulate prey base by feeding on dogs found in the surrounding areas and by scavenging on buffalo carcasses (Edgaonkar & Chellam, Reference Edgaonkar and Chellam2002).
In spite of the higher frequency of goats in the leopard scats we analysed, the relative biomass of cattle was higher, a result of the greater weight of cattle. A similar finding was made in the study of leopard diet in Sri Lanka (Mukenhirn & Eisenberg, Reference Mukenhirn, Eisenberg and Eaton1973). In our study the leopards were probably feeding on discarded carcasses of calves as the Rajasthan Forest Department does not have any records of claims for compensation for predation of cattle.
The fact that leopards prey on the most easily available species (the most numerous), has already been demonstrated (Schaller, Reference Schaller1967; Seidensticker, Reference Seidensticker1983; Kshettry et al. Reference Kshettry, Vaidyanathan and Athreya2018). With a diet dominated by domestic dogs and cats rather than by domestic livestock, the potential economic impact of the leopards of Jhalana Reserve Forest is lower compared to areas where predation of livestock is high and there is a consequent lack of tolerance for such damage (cf. Margulies & Karanth, Reference Margulies and Karanth2018). In addition a large proportion of the rural population surrounding Jhalana Reserve Forest are Jains, who practise the conservation of biodiversity, and especially of large felids (Athreya et al., Reference Athreya, Pimpal, Borkar, Surve, Chakravarty and Ghosalka2018); this facilitates human–leopard coexistence (Kumbhojkar et al., Reference Kumbhojkar, Yosef, Benedetti and Morelli2019).
Domestic dogs are a public health problem in India and elsewhere (Reece & Chawla, Reference Reece and Chawla2006) and occasionally attack other small domestic animals (Srinivasan, Reference Srinivasan2013). For people, dog bites can result in infection, disfigurement, incapacity, post-traumatic stress, and even death, and bites from infected dogs account for > 90% of rabies cases (Seligsohn, Reference Seligsohn2014; Taylor et al., Reference Taylor, Wallace, Balaram, Lindemayer, Eckery and Mutonono-Watkiss2017). Only 2% of the total dog population in the study area are vaccinated (J. Reece, pers. comm., 2019; Reece & Chawla Reference Reece and Chawla2006). Dogs could also transfer infectious diseases to leopards. Canine distemper virus is the second most common cause of death in domestic dogs (Deem et al., Reference Deem, Spelman, Yates and Montali2000) and is also responsible for disease outbreaks amongst the African lion Panthera leo in the Serengeti, the Amur tiger Panthera tigris altaica in the Russian Far East and the Asiatic lion in India (Roelke-Parker et al., Reference Roelke-Parker, Munson, Packer, Kock, Cleaveland and Carpenter1996; Seimon et al., Reference Seimon, Miquelle, Chang, Newton, Korotkova and Ivanchuk2013; ICMR, 2018). Leopards that prey substantially on domestic dogs, as do those of Jhalana Reserve Forest, could be considered as suppliers of a service to the human population amongst whom they thrive, although this potentially exposes the leopards to the canine distemper virus.
Acknowledgements
We thank Sudharshan Sharma, Deputy Conservator of Forests, for his guidance, the Rajasthan Forest Department for permission to conduct research on the leopards of Jhalana and for collecting scat and biological samples; Pratiksha Vedpathak, Benazir Bagwan, Aseem Shendye and Abhay Shendye from Swasti Agro and Bio-Products Pvt. Ltd. for technical support and assistance with scat analysis; Satish Pande, Satish Karmalkar, Pramod Deshpande, Sanjay Khatavkar and Rajkumar Pawar of Ela Foundation for provision of equipment and laboratory facilities for scat analysis; Rahul Lonkar; Bablu Gurjar, Abhishek Gurjar, Dayal Gurjar, Shubham Saini, Varad Bansod and Hrishikesh Wagh for help with field surveys, scat collection and washing of the scats; G.V. Reddy, Head of Forest Office, Rajasthan Forest Department arranged training at Sanjay Gandhi National Park, Mumbai, for scat analysis; Vidhya Athreya, Nikit Surve and his team of volunteers for training in the field; and Abhinav Mehta for drafting Fig. 1.
Author contributions
Study design, fieldwork: SK, RY; data analyses, writing: all authors.
Conflicts of interest
None.
Ethical standards
This research do not involve handling of animals and otherwise abided by the Oryx guidelines on ethical standards. SK and RY worked under research permit 171 of the Rajasthan Forest Department.








