Introduction
A reduction of sleep time has become common in recent years, guided by the demands and opportunities of modern societyReference Spiegel, Knutson, Leproult, Tasali and Van Cauter1. Over the last 40 years, self-reported sleep duration has decreased by 1·5–2 h in the USAReference Kripke, Simons, Garfinkel and Hammond2, 3. The proportion of young adults with a period of sleep shorter than 7 h per d has increased from 15·6 % in 1960 to 37·1 % in 2001–2Reference Kripke, Simons, Garfinkel and Hammond2, 3.
Recent studies show that the alteration in sleep time can influence various aspects associated with the nutritional and metabolic balance of the body, such as the control of body massReference Vioque, Torres and Quiles4–Reference Taheri, Lin, Austin, Young and Mignot7 and the controls of food intakeReference Knutsson8–Reference Scheen10, glycaemic levelsReference Spiegel, Knutson, Leproult, Tasali and Van Cauter1, Reference Van Cauter, Blackman, Roland, Spire, Refetoff and Polonsky11, Reference Gottlieb, Punjabi, Newman, Resnick, Redline, Baldwin and Nieto12 and of the levels of cholesterol and TAGReference Knutsson13–Reference Ghiasvand, Heshmat, Golpira, Haghpanah, Soleimani, Shoushtarizadeh, Tavangar and Larijani15.
Numerous studies have used different methodologies to understand the effects of sleep loss. Sleep deprivation can be total, when no sleep is allowed, or partial, when the retiring time is delayed or the rising time is advanced. In addition, deprivation can last for one or more nights. Results might depend upon the exact nature of the deprivation that is used. Some studies have used shift workers, for example, who might sleep less than day workers, under 5 h on working daysReference Bliwise16. These members of the population have been the main focus of scientific work considering the relationship between sleep and nutrition. Not only might such decreased hours of sleep modify eating behaviour significantlyReference Karlsson, Knutsson, Lindahl and Alfredsson14, Reference Birketvedt, Florholmen, Sundsfjord, Osterud, Dinges, Bilker and Stunkard17–Reference Ishizaki, Morikawa, Nakagawa, Honda, Kawakami, Haratani, Kobayashi, Araki and Yamada22 but also it has been known for some time that the eating habits of night workers during the night shift are alteredReference Waterhouse, Akerstedt, Lennernas and Arendt23, Reference Waterhouse, Buckley, Edwards and Reilly24.
Individuals that sleep less, including shift workers, have been associated in the longer term with a higher propensity for the development of nutritional problemsReference Lennernas, Akerstedt and Hambraeus25, Reference van Amelsvoort, Schouten and Kok26, such as obesity and altered metabolism of foodReference Karlsson, Knutsson, Lindahl and Alfredsson14, Reference Birketvedt, Florholmen, Sundsfjord, Osterud, Dinges, Bilker and Stunkard17–Reference Ishizaki, Morikawa, Nakagawa, Honda, Kawakami, Haratani, Kobayashi, Araki and Yamada22, Reference Waterhouse, Buckley, Edwards and Reilly24, Reference Svatikova, Wolk, Gami, Pohanka and Somers27, dyslipidaemiasReference Lennernas, Akerstedt and Hambraeus25, Reference Romon, Nuttens, Fievet, Pot, Bard, Furon and Fruchart28 and diabetesReference Gottlieb, Punjabi, Newman, Resnick, Redline, Baldwin and Nieto12, Reference Ayas, White, Manson, Stampfer, Speizer, Malhotra and Hu29–Reference Nilsson, Roost, Engstrom, Hedblad and Berglund31. In laboratory studies of healthy young adults submitted to recurrent partial sleep restriction, marked alterations in metabolism, including decreased glucose tolerance and insulin sensitivityReference Spiegel, Leproult and Van Cauter32 and altered metabolism of foodReference Spiegel, Tasali, Penev and Van Cauter33, have been demonstrated.
Given the need for a better understanding of the nutritional problems resulting from alterations in sleep patterns, the present article discusses the influence of sleep on nutritional and metabolic parameters.
Obesity and sleep
Landmark studies by Rechtschaffen et al. Reference Rechtschaffen, Bergmann, Everson, Kushida and Gilliland34 reported that rats submitted to total sleep deprivation (by the disk-over-water method) markedly increased food intake but, nevertheless, lost weight. Many other studies have confirmed these resultsReference Everson, Bergmann and Rechtschaffen35–Reference Papakonstantinou, Ryan and Harris40. Recently, however, Martins et al. Reference Martins, D'Almeida, Nobrega and Tufik41 introduced different procedures to allow accurate estimation of food spillage before, during, and after 120 h of sleep deprivation. Their main finding was that, once corrected for spillage, food intake was not significantly increased during sleep deprivation, even though weight loss did occur during the sleep-deprivation period.
In human subjects, recent studies have pointed to a possible involvement of changed sleep hours in altered energy balance of the body and to alterations in the sleep pattern as a contributory factor to increased obesityReference Spiegel, Knutson, Leproult, Tasali and Van Cauter1, Reference Svatikova, Wolk, Gami, Pohanka and Somers27, Reference Flier and Elmquist42. Many recent studies correlate the short duration of sleep with the increase in the BMI, in adultsReference Vioque, Torres and Quiles4, Reference Kripke, Garfinkel, Wingard, Klauber and Marler6, Reference Taheri, Lin, Austin, Young and Mignot7, Reference Bonnet and Arand43–Reference Vorona, Winn, Babineau, Eng, Feldman and Ware45, childrenReference Padez, Mourao, Moreira and Rosado46–Reference Chaput, Brunet and Tremblay48 and adolescentsReference Gupta, Mueller, Chan and Meininger49–Reference Chen, Wang and Jeng51. In a prospective single-age cohort study with 496 young adults, Hasler et al. Reference Hasler, Buysse, Klaghofer, Gamma, Ajdacic, Eich, Rossler and Angst44 showed an association between short sleep duration and obesity and a negative association between sleep duration and BMI. These associations persisted after controlling for a variety of potentially confounding variables. Reilly et al. Reference Reilly, Armstrong, Dorosty, Emmett, Ness, Rogers, Steer and Sherriff47 found, in 8234 children aged 7 years, that sleep duration in the children when aged 30 months was independently associated with the prevalence of obesity at the age of 7 years. Children showing the lowest two quartiles of sleep duration ( < 10·5 h and 10·5–10·9 h, respectively) were more likely to be obese at age 7 than children in the highest quartile (>12 h).
However, although data from prospective studies and supporting this link are emerging, most of the studies showing an association between short sleeps and obesity have been cross-sectional and do not prove causality. In an attempt to understand better the effect of sleep loss on food intake in man, studies have used models of shift work or jet lag (alterations resulting from rapid crossing of time zones), both of which are situations that alter the sleep pattern and are also associated with alterations in the pattern of food intakeReference Karlsson, Knutsson, Lindahl and Alfredsson14, Reference Birketvedt, Florholmen, Sundsfjord, Osterud, Dinges, Bilker and Stunkard17–Reference Ishizaki, Morikawa, Nakagawa, Honda, Kawakami, Haratani, Kobayashi, Araki and Yamada22, Reference Waterhouse, Kao, Edwards, Atkinson and Reilly52. Some studies have reported that obesity tends to occur more frequently in association with shift work than with daytime-only workReference Karlsson, Knutsson, Lindahl and Alfredsson14, Reference Karlsson, Knutsson and Lindahl18, Reference Theorell and Akerstedt53–Reference Nagaya, Yoshida, Takahashi and Kawai56. During night workReference Waterhouse, Buckley, Edwards and Reilly24, Reference Reilly, Waterhouse and Atkinson57 and after a time-zone transitionReference Waterhouse, Buckley, Edwards and Reilly24, Reference Waterhouse, Minors and Redfern58, there might be additional problems due to the lack of palatable foodReference Waterhouse, Jones, Edwards, Harrison, Nevill and Reilly59. Altered eating habits are a source of concern in night workers, who tend to ‘nibble’ their way through crisps and chocolate bars during the night shift rather than eat a healthy and substantial meal in the middle of itReference Reinberg, Migraine, Apfelbaum, Brigant, Ghata, Vieux and Laporte60–Reference Lennernas, Hambraeus and Akerstedt62. Waterhouse et al. Reference Waterhouse, Kao, Edwards, Weinert, Atkinson and Reilly63 analysed the transient changes in the pattern of food intake following a simulated time-zone transition. Subjects showed significant changes in their pattern of food intake. The distribution of daytime meals was significantly affected on the first post-shift day, with a redistribution of the times that the main, hot meals were eaten.
Even though the mechanisms involved in changed eating habits are not completely understood, it is known that alterations in the sleep–wake schedule affect intracellular circadian clocks – molecular mechanisms that enable the cell, tissue or organism to anticipate diurnal variations in the environment. The environment (of cells and tissues) may include circulating levels of nutrients (for example, glucose, fatty acids and TAG) and various hormones (for example, insulin, leptin, ghrelin, glucocorticoids). As such, alterations in the timing mechanism are likely to induce nutritional changes that may potentiate disrupted metabolismReference Bray and Young64 and influence appetite, satiety and, therefore, food intakeReference Spiegel, Tasali, Penev and Van Cauter33. It is believed also that problems in adjustment of the biological clock, so impairing the duration and quality of sleep, can also modify food intakeReference Lennernas, Akerstedt and Hambraeus25, Reference Waterhouse, Minors and Redfern58, Reference Rutenfranz, Knauth, Fisher, Rutenfranz, Knauth and Fisher65.
Therefore, we will approach more precisely the mechanisms by which the sleep loss can lead to the increase of food intake and obesity.
The role of leptin and ghrelin in the control of food intake and sleep
Eating and sleeping are two kinds of behaviour that are essential for the survival of man and higher animals. Whereas it is obvious that these two processes cannot occur at exactly the same time, there appear to be common regulators of both phenomenaReference Steiger66. With the identification of ghrelin as the endogenous ligand of the growth hormone (GH) secretagogue receptorReference Kojima, Hosoda, Date, Nakazato, Matsuo and Kangawa67, a new endogenous regulator of food intake and, possibly, also of sleep was found. Later, Bodosi et al. Reference Bodosi, Gardi, Hajdu, Szentirmai, Obal and Krueger68 described a relationship between sleep, feeding and ghrelin and their antagonist in energy balance, leptin.
From these findings, many studies have clearly indicated that the reduction in total sleep time is associated with two parallel endocrine behaviours that can significantly alter food intake: the reduction of the anorexigenic hormone leptinReference Taheri, Lin, Austin, Young and Mignot7, Reference Mullington, Chan, Van Dongen, Szuba, Samaras, Price, Meier-Ewert, Dinges and Mantzoros69–Reference Spiegel, Leproult, L'Hermite-Baleriaux, Copinschi, Penev and Van Cauter71 and the increase of the orexigenic hormone ghrelinReference Taheri, Lin, Austin, Young and Mignot7, Reference Spiegel, Tasali, Penev and Van Cauter33, Reference Bodosi, Gardi, Hajdu, Szentirmai, Obal and Krueger68. In individuals who sleep less, this combination of changes results in increased hunger and food intakeReference Spiegel, Tasali, Penev and Van Cauter33. In an experiment carried out by Spiegel et al. Reference Spiegel, Tasali, Penev and Van Cauter33, sleep deprivation in men was associated with an increase of 28 % in ghrelin levels, a reduction of 18 % in the leptin levels and increases of 24 % in hunger and 23 % in appetite (Fig. 1).

Fig. 1 Effect of sleep duration on daytime leptin levels (A), ghrelin levels (B), hunger (C) and appetite (D). (A) Daytime (09.00 to 21.00 hours) profiles of leptin after 2 d with 4 h in bed (![]() ) or 2 d with 10 h in bed (—). Mean leptin levels were 18 % lower when sleep was restricted. (B) Daytime (09.00 to 21.00 hours) profiles of ghrelin from nine of the twelve participants after 2 d with 4 h in bed or 2 d with 10 h in bed. Mean ghrelin levels were 28 % higher in the afternoon and early evening (12.00 to 21.00 hours) when sleep was restricted. (C) Ratings of hunger (0–10 cm visual analogue scale) and (D) overall appetite (0–70 cm visual analogue scale) after 2 d with 4 h in bed or 2 d with 10 h in bed. When sleep was restricted, ratings of hunger and overall appetite increased by 24 and 23 %, respectively. Values are means, with their standard errors represented by vertical bars. (From Spiegel et al. Reference Spiegel, Leproult, L'Hermite-Baleriaux, Copinschi, Penev and Van Cauter71; used with permission from the Annals of Internal Medicine.)
) or 2 d with 10 h in bed (—). Mean leptin levels were 18 % lower when sleep was restricted. (B) Daytime (09.00 to 21.00 hours) profiles of ghrelin from nine of the twelve participants after 2 d with 4 h in bed or 2 d with 10 h in bed. Mean ghrelin levels were 28 % higher in the afternoon and early evening (12.00 to 21.00 hours) when sleep was restricted. (C) Ratings of hunger (0–10 cm visual analogue scale) and (D) overall appetite (0–70 cm visual analogue scale) after 2 d with 4 h in bed or 2 d with 10 h in bed. When sleep was restricted, ratings of hunger and overall appetite increased by 24 and 23 %, respectively. Values are means, with their standard errors represented by vertical bars. (From Spiegel et al. Reference Spiegel, Leproult, L'Hermite-Baleriaux, Copinschi, Penev and Van Cauter71; used with permission from the Annals of Internal Medicine.)
Leptin is a protein composed of 167 amino acids, and it is produced mainly by the adipose tissueReference Reseland, Anderssen, Solvoll, Hjermann, Urdal, Holme and Drevon72. Leptin provides the regulating centre in the brain with information about energy balance, and its release is associated with the promotion of satietyReference Ahima, Prabakaran, Mantzoros, Qu, Lowell, Maratos-Flier and Flier73–Reference Elefteriou, Ahn, Takeda, Starbuck, Yang, Liu, Kondo, Richards, Bannon, Noda, Clement, Vaisse and Karsenty80. Elevated leptin levels at times of metabolic excess activate an anorexigenic pathway, the peptide precursor pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript peptide (CART), and reduce activity in orexigenic pathways, neuropeptide Y (NPY) and agouti-related peptide (AgRP). Low leptin levels, occurring at times of nutrient deficit, result in a reduction of inhibitory influences on NPY/AgRP neurons, a lack of activation of POMC/CART-containing neurons and an overall increase in orexigenic signallingReference Marx81, Reference Moran, Aja and Ladenheim82 (see Fig. 2).
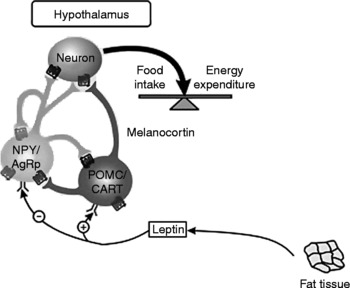
Fig. 2 Central control of food intake. Leptin stimulates pro-opiomelanocortin/cocaine- and amphetamine-regulated transcript peptide (POMC/CART) neurons and inhibits neuropeptide Y (NPY) and agouti-related peptide (AgRP) neurons. The result of these opposing actions is the stimulation of food intake and energy expenditure. (Adapted from Gale et al. Reference Gale, Castracane and Mantzoros229.)
Recent studies with animals have suggested that leptin might participate in the regulation of sleep, systematically reducing rapid eye movement (REM) sleep and influencing non-REM sleepReference Sinton, Fitch and Gershenfeld83. Other work has postulated a direct influence of leptin release on sleep, since the levels of this hormone are higher during sleep than when awakeReference Simon, Gronfier, Schlienger and Brandenberger84. Some evidence suggests that this nocturnal increase is partly a response to the intake of food that took place during the dayReference Schoeller, Cella, Sinha and Caro85. It is believed, however, that sleep per se can affect the regulation of leptin, since studies have shown that the elevation observed during sleep persists in subjects receiving continuous enteral nutrition, even when sleep occurs during the daytimeReference Spiegel, Leproult, L'Hermite-Baleriaux, Copinschi, Penev and Van Cauter71, Reference Simon, Gronfier, Schlienger and Brandenberger84.
Evidence from other laboratory studies has shown that both chronic, partial sleep deprivationReference Spiegel, Leproult, Tasali, Penev and Van Cauter70 and acute sleep deprivationReference Mullington, Chan, Van Dongen, Szuba, Samaras, Price, Meier-Ewert, Dinges and Mantzoros69 might cause a reduction in the serum concentration of leptin. Spiegel et al. Reference Spiegel, Leproult, L'Hermite-Baleriaux, Copinschi, Penev and Van Cauter71 evaluated the pattern of leptin secretion in eleven male individuals subjected to a shortened sleep time (4 h) for six nights. Mean and maximum values of leptin were lower (by 19 and 26 %, respectively) during sleep restriction, compared with the same individuals when they had normal sleep (8 h), suggesting that sleep plays an important role in leptin secretion. Sleep restriction seems to change the capacity of leptin to respond to the body's energy balance and to produce the satiety signal when the energy needs have been adequately metReference Spiegel, Leproult, L'Hermite-Baleriaux, Copinschi, Penev and Van Cauter71. Also, Taheri et al. Reference Taheri, Lin, Austin, Young and Mignot7 observed, in a cross-sectional study carried out with 1024 volunteers, that short sleep was associated with low leptin levels, with a decrease of 15·5 % predicted for habitual sleeps of 5 v. 8 h. Chaput et al. Reference Chaput, Despres, Bouchard and Tremblay86 found a cross-sectional association between short sleep duration and leptin levels in a sample of 323 men and 417 women aged 21–64 years. When compared with adults reporting 7–8 h of sleep per d, and after adjustment for age, sex, and physical activity level, the adjusted OR for overweight or obesity was 1·38 (95 % CI 0·89, 2·10) for those with 9–10 h of sleep and 1·69 (95 % CI 1·15, 2·39) for those with 5–6 h. However, all of these significant differences disappeared after statistical adjustment for plasma leptin levels.
An impact of sleep duration on leptin levels could involve several mechanisms. Considering that leptin release is inhibited by the sympathetic nervous systemReference Rayner and Trayhurn87, another possibility is that sleep restriction results in a reduction in leptin levels due to increased sympathetic activityReference Spiegel, Leproult, L'Hermite-Baleriaux, Copinschi, Penev and Van Cauter71. Alterations in the regulation of cortisol and sympatho–vagal balance, the two most important neurobiological markers of stress, were clear when individuals were studied for 6 d of sleep restrictionReference Spiegel, Leproult, L'Hermite-Baleriaux, Copinschi, Penev and Van Cauter71. A negative association between changes in leptin levels and cortisol during sleep restriction is well documented in the literature, possibly indicating a suppressive effect of leptin on the hypothalamic–pituitary–adrenal (HPA) axisReference Newcomer, Selke, Melson, Gross, Vogler and Dagogo-Jack88–Reference Flier90.
A parallelism between the diurnal and pulsatile variations in thyroid-stimulating hormone (TSH) and leptin levels has been reported in healthy young adultsReference Mantzoros, Ozata and Negrao91. Spiegel et al. Reference Spiegel, Leproult, L'Hermite-Baleriaux, Copinschi, Penev and Van Cauter71 observed a positive association between 24 h variations in leptin and TSH after sleep restriction, which provides compelling evidence for a role for leptin in the physiological regulation of the thyrotropic axis. Evidence suggests that TRH neurons may be regulated by leptinReference Flier90, and a stimulatory effect of leptin on TSH release has been suggested in manReference Mantzoros, Ozata and Negrao91–Reference Ortiga-Carvalho, Oliveira, Soares and Pazos-Moura93 and shown in rodents. In contrast, many other studies have reported negative findings in the role of physiological concentrations of thyroid hormones on leptin regulationReference Spiegel, Leproult, L'Hermite-Baleriaux, Copinschi, Penev and Van Cauter71, Reference Corbetta, Englaro, Giambona, Persani, Blum and Beck-Peccoz94, Reference Kristensen, Pedersen, Langdahl and Richelsen95.
It has been suggested that the reduction in leptin levels after sleep restriction might be an adaptation to increased energy needs, due to the increase in wake timeReference Spiegel, Leproult, L'Hermite-Baleriaux, Copinschi, Penev and Van Cauter71. Studies involving accurate measurements of energy balance in individuals submitted to chronic partial sleep loss are necessary, to rule out the possibility that the state of sleep restriction entails a significant increase in energy expenditure.
A close relationship between leptin and ghrelin, another hormone influenced by sleep, has been described. Ghrelin is a peptide composed of twenty-eight amino acids, produced mainly by the endocrine glands of the stomachReference Kojima, Hosoda, Date, Nakazato, Matsuo and Kangawa67 and duodenumReference Kojima, Hosoda, Date, Nakazato, Matsuo and Kangawa67, and by a number of brain structuresReference Cowley, Smith and Diano96. This hormone increases in periods of fastingReference van der Lely, Tschop, Heiman and Ghigo97, triggering the sensation of hungerReference van der Lely, Tschop, Heiman and Ghigo97–Reference Cummings, Purnell, Frayo, Schmidova, Wisse and Weigle99 in the arcuate nucleusReference Bagnasco, Tulipano, Melis, Argiolas, Cocchi and Muller100, stimulating gastrointestinal motilityReference Masuda, Tanaka, Inomata, Ohnuma, Tanaka, Itoh, Hosoda, Kojima and Kangawa101 and promoting the deposition of lipidsReference Tschop, Smiley and Heiman102. The arcuate nucleus is involved in the central control of food intakeReference Kalra, Dube, Pu, Xu, Horvath and Kalra103, and ghrelin is the only substance that is found endogenously in mammals and that increases hunger and appetite when administered to human subjectsReference Wren, Seal, Cohen, Brynes, Frost, Murphy, Dhillo, Ghatei and Bloom104–Reference Wynne, Giannitsopoulou, Small, Patterson, Frost, Ghatei, Brown, Bloom and Choi109. This hormone contributes to preprandial hungerReference Ogden, Carroll, Curtin, McDowell, Tabak and Flegal110 and plasma concentrations of ghrelin are inversely correlated with the amount of food ingestedReference Bodosi, Gardi, Hajdu, Szentirmai, Obal and Krueger68. Ghrelin is thought to be significantly involved in the neuroendocrine network that regulates energy balance in at least two ways. First, it acts as a peripheral hormone from the stomach that, along with other signals such as insulin or leptin, informs the central energy balance control when energy stores diminish, and also increases orexigenic drive and decreases energy expenditure. Its second involvement is as a hypothalamic neuropeptide, expressed in a previously unidentified population of neurons adjacent to the third ventricle and between the ventromedial hypothalamus, the dorsal hypothalamus, the paraventricular nucleus, and the arcuate nucleus. Efferents of ghrelin-expressing neurons project to key circuits involved in the regulation of central energy balance and may offset the activity of orexigenic neuropeptide Y/Agouti-related protein with anorectic pro-opiomelanocortin neurons and so modulate the output of the efferent pathwayReference van der Lely, Tschop, Heiman and Ghigo97.
Current evidence indicates that ghrelin is also a sleep-promoting factorReference Schussler, Uhr, Ising, Weikel, Schmid, Held, Mathias and Steiger111, inducing slow-wave sleep (SWS) and the nocturnal secretion of GHReference Weikel, Wichniak, Ising, Brunner, Friess, Held, Mathias, Schmid, Uhr and Steiger112. It is well documented that there is an increase in the levels of ghrelin during sleep, followed by a decrease in the morning, some hours before breakfast. The cause of this profile remains to be clarified, since it is anomalous that the levels of a hormone that stimulates hunger are increased during sleep. It has been suggested that ghrelin might produce other metabolic and endocrine functions that remain to be ascertainedReference Weikel, Wichniak, Ising, Brunner, Friess, Held, Mathias, Schmid, Uhr and Steiger112.
As in the case of leptin, sleep seems to influence the pattern of ghrelin secretion, since high levels of this hormone follow the curtailment of sleep in human subjectsReference Spiegel, Tasali, Penev and Van Cauter33. Spiegel et al. Reference Spiegel, Tasali, Penev and Van Cauter33 showed that the curtailment of sleep (to 4 h) in twelve healthy men for a period of 2 d was associated with an increase of almost 28 % in the diurnal levels of ghrelin. Bodosi et al. Reference Bodosi, Gardi, Hajdu, Szentirmai, Obal and Krueger68, in a study with rats, analysed plasma and hypothalamic concentrations of ghrelin before and after sleep deprivation. They observed that levels of hypothalamic ghrelin changed during and after sleep deprivation, increasing during sleep deprivation and decreasing afterwards to levels below baseline. Plasma ghrelin, on the other hand, showed increased levels both duringReference Spiegel, Leproult, L'Hermite-Baleriaux, Copinschi, Penev and Van Cauter71 and after sleep deprivationReference Schussler, Uhr, Ising, Weikel, Schmid, Held, Mathias and Steiger111. Based on this evidence, it has been postulated that high levels of ghrelin in response to sleep deprivation might be a normal response of the body to a greater need for energy intake, as a result of the longer time the individual has remained awake. This hypothesis requires further investigationReference Spiegel, Tasali, Penev and Van Cauter33. Therefore, high ghrelin levels can contribute to an increase of hunger and food intake during sleep loss.
These differences in leptin and ghrelin are likely to increase appetite, possibly explaining the increased BMI observed in individuals with short sleep durationReference Taheri, Lin, Austin, Young and Mignot7. The current literature indicates that the decrease of leptin and increase of ghrelin levels are considered to be the main factors that trigger the increase of hunger when the sleep pattern is alteredReference Spiegel, Tasali, Penev and Van Cauter33. Fig. 3 shows how sleep deprivation might change the pattern of ghrelin and leptin and energy balance.

Fig. 3 Changes in the pattern of ghrelin and leptin release and energy balance produced by sleep deprivation.
Sleep deprivation seems to increase not only appetite but also the preference for foods containing more energyReference Taheri, Lin, Austin, Young and Mignot7, Reference Naitoh113. Spiegel et al. Reference Spiegel, Tasali, Penev and Van Cauter33 showed that the appetite for energy-rich nutrients with high carbohydrate content, including sweets, salty snacks and starchy foods, increased by 33–45 %; by contrast, appetite for fruits, vegetables and high-protein nutrients was less affected. Lennernas et al. Reference Lennernas, Akersted, Hagman, Bruce and Hambraeus114 observed a great preference for the intake of ‘fast food’ and energy-rich snacks during the nocturnal working hours in night workers. The preference for such foods is a source of great concern since, in addition to presenting a hormone pattern that predisposes to an increased energy intakeReference Spiegel, Tasali, Penev and Van Cauter33, individuals with sleep loss (common in night workers) tend to meet this need with foods of low nutritional qualityReference Armstrong115–Reference Sudo and Ohtsuka119. This altered food intake can result from inadequate eating facilities during the night shift but, whatever its cause, it increases the risks of obesityReference van Amelsvoort, Schouten and Kok26, dyslipidaemiasReference Romon, Nuttens, Fievet, Pot, Bard, Furon and Fruchart28 and CVDReference Armstrong115–Reference Sudo and Ohtsuka119.
Sleep duration might represent a major risk factor for the development of weight gain and one that can be modified fairly easily. Unfortunately, most studies that describe hormonal and behavioural changes capable of increasing food intake have been acute interventions, and so it has not been possible to establish what would be their long-term effects. Indeed, there are many other neuropeptides that have stronger effects on food intake, and which have not been measured in sleep-loss studies. Even so, the new studies should lead to a better understanding of the role of sleep in the mechanisms that control hunger and satiety. Also, it is suggested that new studies, to measure the effect of sleep-promoting interventions on appetite and body weight, are required. Even so, the current findings suggest that changes in the levels of leptin and ghrelin, due to sleep curtailment, cause changes in food intake. That is, sleep duration can be added to the environmental factors that are prevalent in our society and that contribute to weight gain and obesity. It might be that a better night's sleep will become a goal in future attempts to combat obesity.
Sleep and energy expenditure
Sleep duration may alter the balance between energy intake and energy expenditure. With regard to energy expenditure, excessive daytime sleepiness and fatigue, resulting from sleep loss (tiredness without increased sleep propensity), have been associated with obesity and have a significant impact on individual wellbeing and public safetyReference Vgontzas, Bixler and Chrousos120. TaheriReference Taheri121 stated that excessive daytime fatigue and sleepiness could contribute to reduced daytime physical activity, which many believe is a major contributor to the current obesity pandemic. KnutsonReference Knutson50 found that about 40 % of 12–16 year olds reported waking up tired; this could have a serious adverse effect on daily physical activity. Additionally, physical activity has a beneficial effect on sleep, suggesting a negative synergy between poor sleep and low physical activity.
Gupta et al. Reference Gupta, Mueller, Chan and Meininger49 studied a tri-ethnic cross-section sample of male and female adolescents, aged 11–16 years (Heartfelt Study). Data obtained from 24 h wrist actigraphy showed that obese adolescents experienced less sleep than non-obese adolescents (P < 0·01). For each hour of lost sleep, the odds of obesity increased by 80 %. Sleep disturbance was not directly related to obesity in the sample, but influenced physical activity levels (P < 0·01). Daytime physical activity diminished by 3 % for every hour increase in sleep disturbance. Other studies, measuring energy expenditure in both sexes, at different ages and following sleep loss, are needed to understand better these relationshipsReference Spiegel, Leproult, L'Hermite-Baleriaux, Copinschi, Penev and Van Cauter71, Reference Rayner and Trayhurn87.
Influence of sleep on glucose metabolism
In man, the homeostatic control of plasma glucose results in a strictly controlled balance between the distribution of glucose (originating in the liver in the post-absorptive state or from the intestine in the postprandial state) and the use of glucose by the tissues such as muscles, adipose tissue and the brain. This control prevents the development of hypoglycaemia or hyperglycaemiaReference Spiegel, Knutson, Leproult, Tasali and Van Cauter1, Reference Van Cauter, Polonsky and Scheen122.
In order to investigate differences in glucose control during sleep and waking periods, a number of recent studies have measured glucose levels of individuals in both statesReference Tiemeier, Pelzer, Jonck, Moller and Rao123. In normal subjects during an overnight sleep, blood levels of glucose remain stable or fall only minimally despite the extended fastReference Van Cauter, Polonsky and Scheen122. By comparison, in subjects awake and fasting in a recumbent position during the daytime period, and in the absence of any physical activity, glucose levels fall by an average of 0·5–1·0 mm (i.e. 100–200 mg/l) over a 12 h periodReference Van Cauter, Polonsky and Scheen122. Thus, a number of mechanisms must operate during nocturnal sleep to maintain stable glucose levels during the fasting periodReference Spiegel, Knutson, Leproult, Tasali and Van Cauter1.
Glucose homeostasis is critically dependent on the ability of pancreatic β-cells to release insulin both acutely (i.e. the acute insulin response to glucose, β-cell responsiveness) and in a sustained fashion, and on the ability of insulin to inhibit hepatic glucose production and promote glucose disposal by peripheral tissues (i.e. insulin sensitivity). Reduced insulin sensitivity, or insulin resistance, occurs when higher levels of insulin are needed to reduce blood glucose levels after the administration of a given amount of exogenous glucoseReference Spiegel, Knutson, Leproult, Tasali and Van Cauter1. Insulin resistance can lead to a marked decrease in glucose tolerance (reflected in higher plasma glucose levels). It is well established that insulin sensitivity, insulin resistance and glucose tolerance vary across the 24 h cycle and can be influenced by a lack of sleepReference Van Cauter, Polonsky and Scheen122.
Recent studies have described a significant impairment in glucose control in individuals who have alterations in their sleep patternReference Spiegel, Knutson, Leproult, Tasali and Van Cauter1, Reference Spiegel, Leproult and Van Cauter32, Reference Mander, Colecchia, Spiegel, Kim, Sannar and Van Cauter124; these subjects are more susceptible to the onset of insulin resistanceReference Spiegel, Knutson, Leproult, Tasali and Van Cauter1, Reference Spiegel, Leproult and Van Cauter32 and type 2 diabetesReference Spiegel, Knutson, Leproult, Tasali and Van Cauter1, Reference Spiegel, Leproult and Van Cauter32, Reference Mikuni, Ohoshi, Hayashi and Miyamura125. Therefore, it is appropriate to address the mechanisms involved in the impaired glucose metabolism by disruption of the sleep–wake rhythm.
Glucose metabolism during sleep
Studies of nocturnal glucose tolerance during sleep – determined by the balance of insulin secretion and insulin action – have used intravenous glucose infusion at a constant rate, or continuous enteral nutrition, and have sampled glucose and insulin without waking the subjectsReference Simon, Brandenberger and Follenius126–Reference Simon, Brandenberger, Saini, Ehrhart and Follenius128.
Van Cauter et al. Reference Van Cauter, Blackman, Roland, Spire, Refetoff and Polonsky11 evaluated glucose and insulin secretion rates in a group of eight normal young men (aged 20–27 years) during constant glucose infusion, including an 8 h period of nocturnal sleep. During nocturnal sleep, levels of glucose and insulin secretion increased by 31 ± 5 and 60 ± 11 %, respectively, and returned to baseline in the morning. During the first half of the sleep period, the increase in plasma glucose was followed by a 50 % increase in insulin secretion. Under these experimental conditions, the major underlying cause of the glucose increase is decreased glucose utilisationReference Van Cauter, Blackman, Roland, Spire, Refetoff and Polonsky11. The profiles of peripheral glucose and insulin concentrations observed in this study confirmed and extended the findings of previous studies, which had shown decreased glucose tolerance in the evening as compared with the morningReference Carroll and Nestel129–Reference Verrillo, De Teresa, Martino, Di Chiara, Pinto, Verrillo, Torello and Gattoni133.
It is estimated that about two-thirds of the fall in glucose utilisation during early sleep is due to a decrease in brain glucose metabolismReference Boyle, Scott, Krentz, Nagy, Comstock and Hoffman134, which is related to the predominance of SWS and associated with a 30–40 % reduction in cerebral glucose metabolism relative to waking values. The remainder of the fall in glucose uptake is thought to reflect decreased peripheral utilisation. Diminished muscle tone during sleep and rapid anti-insulin-like effects of the sleep-onset GH pulseReference Moller, Jorgensen, Schmitz, Moller, Christiansen, Alberti and Orskov135 are both likely to contribute to this decrease in peripheral glucose uptake. During the latter part of the night, glucose tolerance begins to improve, and glucose levels progressively decrease toward morning values, reflecting an increase in glucose uptake. This increase in glucose uptake is partially due to the increases in wakefulness and REM stagesReference Scheen, Byrne, Plat, Leproult and Van Cauter136. Indeed, glucose utilisation during REM sleep and waking is higher than during non-REM sleepReference Boyle, Scott, Krentz, Nagy, Comstock and Hoffman134, Reference Buchsbaum, Gillin, Wu, Hazlett, Sicotte, Dupont and Bunney137–Reference Maquet140. Finally, the latter part of the night appears also to be associated with increased insulin sensitivityReference Van Cauter, Blackman, Roland, Spire, Refetoff and Polonsky11, Reference Plat, Byrne, Sturis, Polonsky, Mockel, Fery and Van Cauter141.
Glucose metabolism during sleep loss
Some evidence has indicated that diabetes is more likely to occur in individuals who experience sleep loss. In a longitudinal study over a 10-year period, Suwazono et al. Reference Suwazono, Sakata, Okubo, Harada, Oishi, Kobayashi, Uetani, Kido and Nogawa142 investigated the effect of alternating shifts on the onset of diabetes mellitus in Japanese workers (n 3203) compared with day-shift workers (n 2426). The OR for the development of diabetes mellitus in the alternating-shift group compared with the day-shift group was 1·35 (95 % CI 1·05, 1·75), indicating that alternating shifts are an independent risk factor for the onset of diabetes mellitus. Morikawa et al. Reference Morikawa, Nakagawa, Miura, Soyama, Ishizaki, Kido, Naruse, Suwazono and Nogawa143 analysed the risk of diabetes mellitus in 2860 men in a factory in Japan over the course of 8 years. They found a significantly increased risk of diabetes mellitus for the two-shift, but not three-shift, system, using white-collar workers as a reference group. More specific studies have examined the relationship between sleep duration and diabetes. Trenell et al. Reference Trenell, Marshall and Rogers144 found the same U-shaped relationship between sleep duration and the incidence of type 2 diabetesReference Ayas, White, Manson, Stampfer, Speizer, Malhotra and Hu29, Reference Yaggi, Araujo and McKinlay145, independent of confounding variables. Analysis of cross-section data from the Sleep Heart Health Study also revealed that reduced sleep duration was associated with an increased prevalence of type 2 diabetes and insulin resistance, after controlling for sleep-disordered breathingReference Gottlieb, Punjabi, Newman, Resnick, Redline, Baldwin and Nieto12, a condition that may also independently influence glucose controlReference Harsch, Schahin, Bruckner, Radespiel-Troger, Fuchs, Hahn, Konturek, Lohmann and Ficker146, Reference Punjabi, Shahar, Redline, Gottlieb, Givelber and Resnick147.
Several studies have shown major changes in glucose tolerance under conditions of sleep restriction or deprivationReference Spiegel, Knutson, Leproult, Tasali and Van Cauter1, Reference Spiegel, Leproult and Van Cauter32, Reference Van Cauter, Polonsky and Scheen122, Reference Grunstein, Stenlof, Hedner and Sjostrom148–Reference Nieto, Young, Lind, Shahar, Samet, Redline, D'Agostino, Newman, Lebowitz and Pickering150. In a laboratory study, Spiegel et al. Reference Spiegel, Leproult and Van Cauter32 analysed the glucose tolerance (measured by an intravenous bolus of glucose; 300 mg/kg body mass) in eleven young men after time in bed had been restricted to 4 h per night for six nights. The authors compared the sleep-debt condition with measurements taken at the end of a sleep-recovery period (fully rested condition) when participants had been allowed 12 h in bed per night for six nights. They observed that glucose tolerance was lower in the sleep-loss condition than in the fully rested condition. Sookoian et al. Reference Sookoian, Gemma, Fernandez Gianotti, Burgueno, Alvarez, Gonzalez and Pirola151 studied 877 day workers and 474 rotating-shift workers. In comparison with day workers, rotating-shift workers had elevated fasting insulin and an increased homeostasis index, which is a measure of insulin resistance.
To define the roles of circadian rhythmicity (intrinsic effects of time of day, independent of the sleep or wake condition) and sleep (intrinsic effects of the sleep condition, irrespective of the time of day) on the 24 h variation in glucose tolerance, Van Cauter et al. Reference Van Cauter, Blackman, Roland, Spire, Refetoff and Polonsky11 evaluated glucose and insulin secretion rates during a 53 h period − 8 h of nocturnal sleep, followed by 28 h of sleep deprivation including a period of nocturnal sleep deprivation, and then 8 h of daytime recovery sleep. During sleep deprivation, glucose levels and insulin secretion rose to reach a maximum at a time corresponding to the beginning of the habitual sleep period. The magnitude of the rise above morning levels averaged 17 (sd 5) % for glucose and 49 (sd 8) % for calculated insulin secretion. Serum insulin levels did not parallel the circadian variation in insulin secretion, indicating the existence of an approximate 40 % increase in insulin clearance during the night. Daytime sleep was associated with a 16 (sd 3) % rise in glucose levels, a 55 (sd 7) % rise in insulin secretion and a 39 (sd 5) % rise in serum insulin. The profiles observed under these conditions indicate unequivocally that both circadian rhythmicity and sleep modulate glucose regulationReference Van Cauter, Blackman, Roland, Spire, Refetoff and Polonsky11 (Fig. 4).

Fig. 4 Profiles of glucose (A) and insulin secretion rates (ISR) (B) in a group of eight normal young men (aged 20–27 years) studied during a 53 h period including 8 h of nocturnal sleep (■), followed by 28 h of sleep deprivation including a period of nocturnal sleep deprivation (![]() ) and 8 h of daytime recovery sleep (
) and 8 h of daytime recovery sleep (![]() ). Data were obtained at 20 min intervals under continuous glucose infusion. Values are means, with their standard errors represented by vertical bars. (Adapted from Van Cauter et al. Reference Van Cauter, Blackman, Roland, Spire, Refetoff and Polonsky11; cited by Spiegel et al. Reference Spiegel, Knutson, Leproult, Tasali and Van Cauter1; used with permission from the Journal of Applied Physiology.)
). Data were obtained at 20 min intervals under continuous glucose infusion. Values are means, with their standard errors represented by vertical bars. (Adapted from Van Cauter et al. Reference Van Cauter, Blackman, Roland, Spire, Refetoff and Polonsky11; cited by Spiegel et al. Reference Spiegel, Knutson, Leproult, Tasali and Van Cauter1; used with permission from the Journal of Applied Physiology.)
Further studies are necessary to evaluate whether there is a difference in glucose metabolism following intravenous infusion or oral intake of glucose or other kinds of carbohydrates under conditions of sleep deprivation. It is also important that the impact of chronic sleep debt be clarifiedReference Spiegel, Knutson, Leproult, Tasali and Van Cauter1. Mander et al. Reference Mander, Colecchia, Spiegel, Kim, Sannar and Van Cauter124 observed that healthy individuals of both sexes, whose sleep had been curtailed (to less than 6·5 h per night) for a minimum period of 6 months, had a response to intravenous glucose similar to that of individuals who had slept longer (7·5–8·5 h), but at the cost of having markedly higher insulin secretion. This finding suggests that there might be a mechanism of metabolic adaptation when sleep debt becomes chronic. If this is the case, the initial impairment to glucose tolerance and to the responsiveness of β-cells might foster the subsequent development of insulin resistanceReference Spiegel, Knutson, Leproult, Tasali and Van Cauter1.
There are other explanations for the changes in glucose metabolism during conditions of sleep loss. Cortisol, whose 24 h rhythm is noteworthy for its robustness and persistence under a large variety of pathological conditions, is a hormone that plays an important role in glucose metabolism. A modest elevation in cortisol levels during the night was present in elderly and adult individuals who had been sleep deprivedReference van Coevorden, Mockel, Laurent, Kerkhofs, L'Hermite-Baleriaux, Decoster, Neve and Van Cauter152–Reference Leproult, Copinschi, Buxton and Van Cauter155. In both groups, the nocturnal elevation of cortisol could reflect an impairment of feedback inhibition on the HPA axisReference Plat, Leproult, L'Hermite-Baleriaux, Fery, Mockel, Polonsky and Van Cauter156.
Spiegel et al. Reference Spiegel, Leproult and Van Cauter32 observed, in eleven young men after time in bed had been restricted to 4 h per night for six nights (sleep-debt condition), that the evening cortisol concentrations were raised (P = 0·0001) and the activity of the sympathetic nervous system was increased (P < 0·02). The sleep-debt condition, compared with the sleep-recovery condition (12 h in bed per night for six nights), was associated with alterations in the 24 h profile of plasma cortisol, including a shorter quiescent period and raised concentrations in the afternoon and early evening (P = 0·0001). This latter disturbance may reflect decreased efficacy of the negative-feedback regulation of the HPA axis.
Cortisol has an immediate effect on the secretion of insulin, producing an inhibition in the absence of changes in glucose concentrationReference Plat, Byrne, Sturis, Polonsky, Mockel, Fery and Van Cauter141. This effect has been demonstrated in both in vitro Reference Billaudel and Sutter157–Reference Lambillotte, Gilon and Henquin162 and in vivo studiesReference Kalhan and Adam163–Reference Boden, Ruiz, Urbain and Chen166.
One of the slower effects of a rise in cortisol levels is the onset of insulin resistance 4–6 h afterwardsReference Plat, Leproult, L'Hermite-Baleriaux, Fery, Mockel, Polonsky and Van Cauter156. Therefore, the normal nocturnal elevation of cortisol levels might adversely affect glucose regulation during the night and the following day. In the long term, it might contribute to age-related reductions in glucose tolerance and insulin sensitivity. The hypothesis also suggests that the normal elevation of plasma cortisol at night, when the HPA axis is normally inhibited, would result in deleterious metabolic effects that are stronger than those that take place due to a similar elevation during the morning, when the HPA axis is fully activatedReference Plat, Leproult, L'Hermite-Baleriaux, Fery, Mockel, Polonsky and Van Cauter156. A slow reduction of cortisol concentration in the afternoon is consistent with altered hippocampal mechanisms that control the negative feedback upon the HPA axisReference Spiegel, Leproult and Van Cauter32. On the other hand, some studies rule out the possibility that the circadian variations in cortisol concentrations contribute to the diurnal variation in glucose tolerance, since this tolerance is higher in the morning (when cortisol levels are high) and lower in the first half of the night (when cortisol levels are low)Reference Jarrett and Krieger130, Reference Dinneen, Alzaid, Miles and Rizza165. The coincidences of increased insulin sensitivity with high cortisol levels in the morning, and of decreased insulin sensitivity with low cortisol levels in the evening, appear to contradict the well-known adverse effects of glucocorticoids on insulin sensitivity. However, this interpretation is based on the assumption that alterations of insulin resistance are an immediate consequence of changes in cortisol concentrationsReference Van Cauter, Polonsky and Scheen122.
Disorders in the profile of GH secretion might also contribute to the alterations in glucose regulation observed during sleep loss. GH is secreted in a series of pulses throughout the whole 24 h cycle, with greater changes in concentration, due to more frequent and larger secretory pulses, taking place during sleepReference Takahashi, Kipnis and Daughaday167. In normal adults, peak plasma concentrations of GH take place during the first half of sleep, in association with the time of most SWSReference Takahashi, Kipnis and Daughaday167–Reference Van Cauter, Plat and Copinschi170. The amount of GH secreted during the first episode of SWS is quantitatively related to the durationReference Holl, Hartman, Veldhuis, Taylor and Thorner171, Reference Van Cauter, Kerkhofs, Caufriez, Van Onderbergen, Thorner and Copinschi172 and the intensityReference Gronfier, Luthringer, Follenius, Schaltenbrand, Macher, Muzet and Brandenberger173 of the SWS. The rapid anti-insulin-like effects of the GH pulseReference Moller, Jorgensen, Schmitz, Moller, Christiansen, Alberti and Orskov135 are responsible for reducing glucose uptake by the peripheral tissuesReference Spiegel, Knutson, Leproult, Tasali and Van Cauter1.
Considering the importance and the multiplicity of the metabolic actions of GH, even when there are only minor changes in the secretion profile over the course of the 24 h, these could be associated with significant peripheral effectsReference Spiegel, Leproult, Colecchia, L'Hermite-Baleriaux, Nie, Copinschi and Van Cauter174. Plat et al. Reference Plat, Leproult, L'Hermite-Baleriaux, Fery, Mockel, Polonsky and Van Cauter156 showed that sleep restriction was associated with a longer elevation of GH and an increase in cortisol levels during the night. Sleep-onset GH secretion is thought to facilitate the maintenance of stable overnight glucose levels despite the prolonged fasting conditionReference Van Cauter, Polonsky and Scheen122. Indeed, studies that have used intravenous administrations of a low dose of synthetic GH to mimic physiological pulsatile release have shown that a primary effect is a rapid decrease in muscular glucose uptakeReference Moller, Jorgensen, Schmitz, Moller, Christiansen, Alberti and Orskov135, Reference Moller, Butler, Antsiferov and Alberti175. Spiegel et al. Reference Spiegel, Leproult, Colecchia, L'Hermite-Baleriaux, Nie, Copinschi and Van Cauter174 evaluated a semi-chronic partial sleep loss (sixteen consecutive nights in the clinical research centre, including three nights with 8 h bedtime from 23.00 to 07.00 hours, six nights with bedtime limited to a 4 h period from 01.00 to 05.00 hours, and seven nights with 12 h bedtime from 21.00 to 09.00 hours) on the 24 h GH profile. Eleven young men were studied after six nights of restricted bedtimes (01.00 to 05.00 hours) and after seven nights of extended bedtimes (21.00 to 09.00 hours, the fully rested condition). After 1 week of sleep restriction, the biphasic nature of nocturnal GH release resulted in an extended period of elevated GH concentration compared with fully rested conditions. This extended exposure of peripheral tissues to higher GH levels may have adversely affected glucose regulationReference Plat, Leproult, L'Hermite-Baleriaux, Fery, Mockel, Polonsky and Van Cauter156.
All these mechanisms suggest an important impairment in glucose metabolism during sleep, especially in individuals submitted to sleep restriction or deprivationReference Spiegel, Leproult and Van Cauter32. With humans spending a significant proportion of their lives asleep, it is not surprising that the body compensates for these periods of enforced fasting by manifesting a degree of peripheral insulin resistance, thereby maintaining circulating glucose levels. Likewise, there appears to be value in maintaining levels of circulating glucose during periods of perceived stress, in order to sustain cognitive and metabolic functionReference Trenell, Marshall and Rogers144.
These results highlight the fact that dietary care is fundamental in those individuals who are more susceptible to glucose metabolic disorders such as diabetes and insulin resistance. Accordingly, the intake of carbohydrates near bedtime should be minimal, since the little evidence that does exist suggests that intake at 22.00 hours entails a considerably stronger insulin and glucose response compared with the same intake at 10.00 hoursReference Hampton176. This response is compatible with the considerable insulin resistance observed during the nightReference Morgan, Hampton, Gibbs and Arendt177. In addition, it has been suggested that the intake of large amounts of food at night, during the circadian phase when there is lowest insulin sensitivity, might generate effects that predispose individuals to the onset of other metabolic disordersReference Morgan, Hampton, Gibbs and Arendt177.
Fat metabolism during sleep
Recent studies have shown that night workers, with chronic sleep loss, are more predisposed to fat metabolism disordersReference Nakamura, Shimai, Kikuchi, Tominaga, Takahashi, Tanaka, Nakano, Motohashi, Nakadaira and Yamamoto9, Reference Karlsson, Knutsson, Lindahl and Alfredsson14, Reference Ghiasvand, Heshmat, Golpira, Haghpanah, Soleimani, Shoushtarizadeh, Tavangar and Larijani15, Reference Romon, Nuttens, Fievet, Pot, Bard, Furon and Fruchart28, Reference Orth-Gomér54, Reference Knutsson, Akerstedt and Jonsson55, Reference Hampton, Morgan, Lawrence, Anastasiadou, Norris, Deacon, Ribeiro and Arendt178, Reference Lasfargues, Vol, Caces, Le Clesiau, Lecomte and Tichet179. These individuals present higher serum levels of TAGReference Nakamura, Shimai, Kikuchi, Tominaga, Takahashi, Tanaka, Nakano, Motohashi, Nakadaira and Yamamoto9, Reference Karlsson, Knutsson, Lindahl and Alfredsson14, Reference Romon, Nuttens, Fievet, Pot, Bard, Furon and Fruchart28, Reference Orth-Gomér54, Reference Knutsson, Akerstedt and Jonsson55, Reference Lasfargues, Vol, Caces, Le Clesiau, Lecomte and Tichet179 and cholesterolReference Karlsson, Knutsson, Lindahl and Alfredsson14, Reference Ghiasvand, Heshmat, Golpira, Haghpanah, Soleimani, Shoushtarizadeh, Tavangar and Larijani15, Reference Hampton, Morgan, Lawrence, Anastasiadou, Norris, Deacon, Ribeiro and Arendt178 compared with day workers (Table 1).
Table 1 Lipid profile disturbances in shift workers

It is widely recognised that environmental factors, especially feeding, are critical for the development of those problems, and night workers have inadequate eating habits that might contribute to these problemsReference Romon, Nuttens, Fievet, Pot, Bard, Furon and Fruchart28, Reference Armstrong115–Reference Moore, Touitou and Haus118. Nevertheless, another body of evidence suggests that the problems might be triggered by metabolic disorders that do not depend on food intakeReference Romon, Nuttens, Fievet, Pot, Bard, Furon and Fruchart28; rather, they are a pathogenic effect induced by a mismatch between circadian rhythms, environmental factors and social stressReference Knutsson and Boggild180. In other words, the difficulties might arise from a clash between the body clock and the environmentReference Weibel and Brandenberger181. Therefore, we shall now consider alterations of fat metabolism that are triggered by disruption of the sleep–wake rhythm.
Circadian control of fat metabolism
The supply of TAG from the adipocytes results from a balance between the uptake and release of NEFA. These fatty acids are formed by the hydrolysis of circulating TAG by the lipoprotein lipase (LPL) enzymeReference Eckel182 and by the lipolysis of TAG into NEFA and glycerol by hormone-sensitive lipaseReference Large, Arner, Reynisdottir, Grober, Van Harmelen, Holm and Langin183. These processes are reciprocally regulated, suggesting an inverse relationship between the activities of LPL and hormone-sensitive lipaseReference Patten184.
Dramatic diurnal variations in adipocyte lipolysis and lipogenesis occur in mammals. When an animal sleeps, rates of lipolysis increase, resulting in increased release of NEFA into the circulation. In contrast, when an animal is awake, rates of lipolysis decrease, with a concomitant increase in lipogenesis. Diurnal variations in adipose TAG turnover have been explained primarily in terms of reciprocal changes in neurohumoral influences promoting lipolysis and lipogenesisReference Bray and Young64. According to the ‘lipogenic–lipolytic’ theory of ArmstrongReference Armstrong115, daytime food intake is associated with glucose metabolism and fat deposition, and nocturnal fasting with fat metabolism. It follows that fat metabolism will be more active during the night and fat oxidation takes place mainly at this timeReference Takahashi, Kipnis and Daughaday167, Reference Parker, McNamara, Brown, Garrigan, Kolb, Batwin and Ahrens185.
Hormones that acutely affect lipolysis in human adipocytes are catecholamines (adrenaline and noradrenaline) and insulinReference Large and Arner186. Circulating GH also plays a fundamental role in the regulation of fat metabolismReference Corpas, Harman and Blackman187, Reference Angelin and Rudling188, generally increasing energy flow in the lipid transportation system by stimulation of lipolysis in adipose tissueReference Davidson189, Reference Lind, Rudling, Ericsson, Olivecrona, Eriksson, Borgstrom, Eggertsen, Berglund and Angelin190. Some authors suggest that GH is the main hormone in the control of lipolysis. Interestingly, peak production of this hormone occurs during the night, suggesting that this might be the pathway through which lipolysis is stimulated during sleepReference Macgorman, Consoli, Jensen and Miles191. In addition to increases in GH concentration, adrenocorticotropinReference Tanaka, Nicholson and Orth192 and prolactinReference Rantz193 also rise and then fall during the night, and have also been implicated in the regulation of lipolysisReference White and Engel194, Reference Winkler, Rathgeb, Steele and Altszuler195. These results support the view that the circadian variations of several endocrines modulate both fat deposition and utilisation during a 24 h period.
Circadian rhythm of lipid tolerance and cholesterol biosynthesis
TAG concentrations in the blood show a circadian variation, with maximum values around 03.00 to 04.00 hours and minimum values at noonReference Rivera-Coll, Fuentes-Arderiu and Diez-Noguera196. Morgan et al. Reference Morgan, Arendt, Owens, Folkard, Hampton, Deacon, English, Ribeiro and Taylor197 observed a marked increase of plasma TAG during the night and its dissociation into two significant components. The first was related to the internal body clock, and the second to the time after waking. This increase in TAG is possibly due to an impairment in lipid tolerance during the night – that is, an impaired postprandial TAG clearanceReference Arasaradnam, Morgan, Wright and Gama198 – in turn due to insufficient insulin activity at this timeReference Rivera-Coll, Fuentes-Arderiu and Diez-Noguera196, Reference Morgan, Arendt, Owens, Folkard, Hampton, Deacon, English, Ribeiro and Taylor197, Reference Romon, Le Fur, Lebel, Edme, Fruchart and Dallongeville199; the result will be a reduction in the activity of LPL and decreased hydrolysis of plasma TAGReference Garrido200. Consequently, concentrations of TAG in the blood will be high during the nightReference Karlsson, Knutsson, Lindahl and Alfredsson14, Reference Garrido200.
Other possible causes of nocturnal lipid intolerance are that the clearance of TAG from the circulation, or the suppression of hepatic synthesis and/or secretion of TAG, is impairedReference Arasaradnam, Morgan, Wright and Gama198. Lemberger et al. Reference Lemberger, Saladin, Vazquez, Assimacopoulos, Staels, Desvergne, Wahli and Auwerx201 state that the α-sub-type hepatic PPAR indirectly influences TAG hydrolysis, and so affects the levels of circulating TAG, via regulation of the synthesis of apo CIII (a lipoprotein fraction that is an inhibitor of LPL).
Studies involving the hepatic lipase enzyme suggest that its activity is positively related to serum concentrations of TAGReference Cohen, Vega and Grundy202 and that hypertriacylglycerolaemia is a characteristic of hepatic lipase deficiencyReference Connelly203. Moreover, this enzyme has been implicated in impairment of the postprandial clearance of lipoproteinsReference Jansen, Breedveld and Schoonderwoerd204. Consequently, it is possible that reduced levels of hepatic lipase at night might also contribute to nocturnal lipid intoleranceReference Arasaradnam, Morgan, Wright and Gama198.
Advances of the sleep–wake cycleReference Ribeiro, Hampton, Morgan, Deacon and Arendt205 and simulated shift workReference Ribeiro, Hampton, Morgan and Arendt206 have both revealed an increase in the postprandial response of TAG in the night. It is known that factors such as the rate of gastric emptying, TAG hydrolysis in the intestine, and intestinal motility might influence the rate of TAG flow into the circulationReference Cohn, McNamara, Krasinski, Russell and Schaefer207, and insulin resistance has also been suggested to be a factor contributing to this increase in postprandial TAGReference Lund, Arendt, Hampton, English and Morgan208. Since LPL has a reduced activity at night and plays an important role in the regulation of postprandial TAG clearanceReference Knapper, Puddicombe and Morgan209, higher levels of TAG are observed after food intake at night compared with the daytimeReference Romon, Le Fur, Lebel, Edme, Fruchart and Dallongeville199.
The decrease of lipid tolerance in the night-time can cause high levels of TAG in the circulation, especially in association with food intake. Traditionally, fasting plasma TAG concentrations have not been recognised as an independent risk factor affecting the pathogenesis and progression of CHD210. However, more recent epidemiological evidence suggests that the relative importance of TAG as a risk factor for CHD may have been underestimated. A large meta-analysis of seventeen population-based prospective studies showed that plasma TAG concentration was an independent risk factor for CHDReference Hokanson and Austin211. This analysis also showed that plasma TAG concentrations were particularly important in relation to CHD risk in women; an increase in plasma TAG concentration increased cardiovascular risk by 76 % in women compared with 32 % in menReference Hokanson and Austin211.
Current evidenceReference Parker, McNamara, Brown, Garrigan, Kolb, Batwin and Ahrens185, Reference Jones and Schoeller212 indicates that the rates of endogenous cholesterol biosynthesis in man are subject to large changes over the course of the dayReference Parker, McNamara, Brown, Garrigan, Kolb, Batwin and Ahrens185, Reference Jones and Schoeller212–Reference Jones, Leitch and Pederson214 and increase at night. The concentration of HDL-cholesterol also shows a circadian variation, which is phased opposite to that of TAG, with minimum values at around 04.00 hours and maximum values around noonReference Rivera-Coll, Fuentes-Arderiu and Diez-Noguera196. MiettinenReference Miettinen213 observed an increase in the cholesterol precursors squalene and lanosterol, with maximum values found between midnight and 04.00 hours. Parker et al. Reference Parker, McNamara, Brown, Garrigan, Kolb, Batwin and Ahrens185 observed a nocturnal increase in plasma levels of mevalonate, a precursor of cholesterol biosynthesis whose production is controlled by hydroxymethylglutaryl-CoA reductaseReference Dietschy and Wilson215, and these were correlated with the rate of cholesterol productionReference Popjak, Boehm, Parker, Edmond, Edwards and Fogelman216–Reference McNamara, Ahrens, Parker and Morrissey218.
The behaviour of cholesterol metabolism in human subjects during sleep deprivationReference Vondra, Brodan, Dobiasova, Vitek and Kopecka219 and rotating-shift systemsReference Theorell and Akerstedt53 has led to the suggestion that changes in the sleep–wake and/or light–dark cycles might be involved. On the other hand, Cella et al. Reference Cella, Van Cauter and Schoeller220 observed that, with alterations in the sleep–wake and/or light–dark cycles but with no changes to meal times, the diurnal pattern of cholesterol synthesis was unaltered; this result shows that the rhythm is more strongly regulated by meal times rather than by the sleep–wake and light–dark cycles. However, in other studies carried out upon animals, both the circadian rhythm and meal times played important roles in the regulation of the diurnal variation of cholesterol synthesisReference Ribeiro, Hampton, Morgan, Deacon and Arendt205. It has also been found that eating at night leads to an increase in the LDL:HDL ratioReference Lennernas, Akerstedt and Hambraeus25.
The increase of GH in SWS might be associated with the increase in cholesterol synthesis during the nightReference Takahashi, Kipnis and Daughaday167, Reference Parker, McNamara, Brown, Garrigan, Kolb, Batwin and Ahrens185. Takahashi et al. Reference Takahashi, Kipnis and Daughaday167 and Parker et al. Reference Parker, McNamara, Brown, Garrigan, Kolb, Batwin and Ahrens185 have suggested that GH might have a direct regulating effect on cholesterol synthesis due to the strong temporal association between the increases of GH and mevalonate in the night. The hypothesis is that β-oxidation, which also is increased during the night, might be the pathway for the oxidation of the NEFA to supply the two carbon fragments necessary to condense and form hydroxymethylglutaryl-CoA, the immediate precursor of mevalonateReference Takahashi, Kipnis and Daughaday167, Reference Parker, McNamara, Brown, Garrigan, Kolb, Batwin and Ahrens185. However, Boyle et al. Reference Boyle, Avogaro, Smith, Bier, Pappu, Illingworth and Cryer221 and Cella et al. Reference Cella, Van Cauter and Schoeller220 observed that an abrupt change in sleep time, with the resulting change in release of GH, was not associated with detectable changes in cholesterol synthesis.
Cella et al. Reference Cella, Van Cauter and Schoeller220 showed that TSH, normally inhibited by nocturnal sleep, had a peak coinciding with the maximum rate of cholesterol synthesis on days with normal amounts of sleep at night; by contrast, a twofold increase in the amplitude of the TSH rhythm was observed during sleep deprivationReference Parker, Rossman, Pekary and Hershman222–Reference Allan and Czeisler224. This major alteration in the profile of TSH concentration was associated with a modest elevation in the peak of the rhythm of cholesterol synthesis, and these simultaneous changes were reflected in an increase in the cross-correlation between themReference Cella, Van Cauter and Schoeller220. These observations support the view that TSH might exert an effect on cholesterol synthesis, mainly during sleep deprivationReference Cella, Van Cauter and Schoeller220. Under normal sleep–wake conditions, the diurnal variation in secretion of thyroid hormone has a low amplitude and its circadian rhythm might go undetectedReference Allan and Czeisler224, Reference Van Cauter, Turek and DeGroot225. During sleep deprivation, by contrast, a nocturnal increase in this hormone parallels an increase of TSHReference Van Cauter, Turek and DeGroot225. Since the activity of the hydroxymethylglutaryl-CoA reductase enzyme is influenced by this thyroid hormoneReference Guder, Nolte and Wieland226, Reference Ness, Dugan, Lakshmanan, Nepokroeff and Porter227, it is conceivable that the activation of the pituitary–thyroid axis during sleep deprivation exerts a modest influence on cholesterol synthesisReference Cella, Van Cauter and Schoeller220.
Therefore, it is possible that the normal increases in levels of TAG and cholesterol at night is accentuated by sleep loss and abnormal nocturnal food intake, thus contributing to the risk of CVD. Many studies show that physiological events that control lipid metabolism are strongly influenced by the sleep–wake cycle. Thus, it is reasonable to suppose that interruption of the sleep–wake cycle, resulting in a decreased sleep time, can impair lipid metabolism. This impairment is demonstrated in studies that show that shift workers, who sleep less than 5 h on their working daysReference Bliwise16, have a greater frequency of disorders of lipid metabolism.
The role of sleep loss in fat metabolism is an exciting new field of study, and is believed to result in an increased incidence of dyslipidaemias. Elucidation of the mechanisms involved might have profound implications for an understanding of these disorders. In the future, it will be necessary to investigate whether these processes have an impact on both susceptibility to dyslipidaemias and on susceptibility to the development of the potentially debilitating co-morbidities associated with disorders of fat metabolism. Nevertheless, it might prove difficult to show unequivocally that there is a causal relationship between sleeps of short duration and problems with lipid metabolism.
Conclusion
We conclude that sleep affects the body's nutritional control, and that alterations to an individual's sleep pattern might stimulate food intake and so contribute to the onset of disorders of glucose and fat metabolism. Sleep loss also contributes to the onset of insulin resistance, type 2 diabetes and obesity, as direct consequences of the influence of sleep on glucose metabolism and of an alteration in feeding behaviour generated by appetite dysregulation. It is also important to acknowledge that the laboratory analyses do not prove causality between sleeps of short sleep duration and diabetes. However, the current experimental literature involves only very small numbers of participants who are nearly all men and young. Experimental evidence from older individuals and from women of all ages is required to confirm that sleep loss causes metabolic problems in the population as a whole. The increase in blood lipids during the night, associated with altered eating patterns, seems to contribute to the onset of dyslipidaemias. Indeed, not only adequate sleep time but also balanced eating habits are of fundamental importance for the maintenance of health, and both should be encouraged by health professionals. In particular, individuals with chronic sleep loss, such as night workers, deserve specific nutritional advice. Further studies are required so that the detailed needs of these individuals can be better understood.
Acknowledgements
C. A. C. was primarily responsible for the literature revision of the study, supervised its execution and reviewed each step of writing. I. Z., M. D. and H. G. P. were jointly responsible for the literature revision of the study. B. E. and J. W. provided assistance in all literature revision and execution of the study and for the revision of the English language. S. T. and M. T. de M. assisted in execution of all parts of the study. We acknowledge AFIP, Sleep Institute, FAPESP, CEDIP/FAPESP (no. 998/14 303-3), CEPE, UNIFESP, CENESP/UNIFESP, FADA, CEMSA, CAPES, CNPQ and FADA/UNIFESP.







