Introduction
The domestic dog (Canis familiaris) is believed to have evolved from the grey wolf (C. lupis) as a separate species at least 15 000 years ago and it is thought to be the first animal species to be domesticated by humansReference Vilà, Savoleinen, Maldonado, Amorim, Rice, Honeycutt, Crandall, Lundeberg and Wayne1, Reference Savolainen, Zhang, Luo, Lundeberg and Leitner2. At the present time, as a result of selective breeding, approximately 400 distinct dog breeds are recognised worldwide, representing a large variation in body size and weight, with the latter ranging from 1 to 90 kg. Initial functions of dogs such as hunting, shepherding and guarding have diminished gradually in importance in favour of the dog's role as a companion to humansReference Hart and Serpell3. Though most human–dog relationships are fulfilling, each year a large number of animals are abandoned by their owners or relinquished to animal sheltersReference Marston and Bennett4. Aggression toward people and animals, running away, destructive behaviour, disobedience, house soiling and excessive barking are unwanted behaviours that make owners relinquish or abandon their dogsReference Salman, Hutchison, Ruch-Gallie, Kogan, New, Kass and Scarlett5. Although only 20 % of the dogs in the US shelters are assigned by their owners for euthanasiaReference Patronek, Glickman, Beck, McCabe and Ecker6, a further 40 % of dogs admitted are euthanisedReference Houpt, Honig and Reisner7. Of the sheltered dogs that are purchased by new owners, approximately 20 % are returned to sheltersReference Patronek, Glickman, Beck, McCabe and Ecker6, Reference Houpt, Honig and Reisner7 and a large proportion of these animals are euthanisedReference Marston and Bennett4. The number of dogs and cats euthanised annually in the USA is estimated to be between 5 and 17 millionReference Carter8, Reference Rowan9, with 3–6 million as a result of behaviour problemsReference Dodman, Shuster, Dodman and Shuster10. Strategies that combat problem behaviours in dogs will greatly benefit animal welfare. The behaviour of individual dogs is controlled by numerous factors and from studies in humans it can be derived that nutrition plays a role also. For example, diets rich in vitamins and minerals may decrease anti-social behaviour in schoolchildrenReference Schoenthaler and Bier11 and supplementation of vitamins, minerals and essential fatty acids decreased anti-social behaviour, including violence, of young adult prisonersReference Gesch, Hammond, Hampson, Eves and Crowder12. Dietary effects on behaviour have been investigated for anti-social aspects, but also for behavioural changes related to ageing and, in this, dogs have been used as a model for humans. Dogs develop similar cognitive deficits and neuropathology as can be seen in ageing humans and elderly suffering from dementiaReference Studzinski, Araujo and Milgram13. Milgram and co-workers initiated a series of experiments with young and aged beagle dogs to study dietary interventions on age-related cognitive decline. Results showed that canine food enriched with antioxidants and mitochondrial cofactors decreased the rate of cognitive decline in aged beagle dogs under laboratory conditions and improved age-related behavioural changes in older pet dogs held in home situations (for reviews, see Roudebush et al. Reference Roudebush, Zicker, Cotman, Milgram, Muggenburg and Head14 and ZickerReference Zicker15). These findings demonstrate clearly that canine behaviour can be influenced by dietary components.
The present review presents an overview of our current knowledge on the influence of dietary macronutrient composition on the behaviour of dogs and explores the underlying mechanisms by which diet may affect behaviour. Findings from food–behaviour studies in dogs and other mammals are integrated to assess in what way problem behaviour in dogs may be reduced through dietary means.
Effects of dietary amino acids and protein content on behaviour
After ingestion, proteins are enzymically degraded and absorbed in the small intestine mainly as tripeptides, dipeptides and free amino acids. After hydrolysis of the peptides in the enterocytes, the free amino acids are transported through the portal vein to the liver. Amino acids are important constituents required for the synthesis of enzymes and other proteins, and used as precursors for the synthesis of neurotransmitters and hormonesReference Massey, Blakeslee and Pitkow16. For example, serotonin, catecholamines, acetylcholine and histamine are metabolites from tryptophan, tyrosine, choline and histidine, respectivelyReference Young17. These neurotransmitter precursors (except for choline) are amino acids and are natural dietary constituents. Behaviour results from signal detection, transmission and processing in the (central) nervous system, which is accomplished and modulated by chemical messengers such as neurotransmitters and hormones. Changes in neurotransmitter precursors such as tryptophan and tyrosine are, therefore, likely to influence behaviour. The amount and timing of food intake, diet composition and digestibility are all factors that determine the availability of different amino acids, i.e. precursors of chemical messengers. Consequently, such factors may influence behaviour. The effects of tryptophan and tyrosine on behaviour will be discussed as these could be relatively potent modulators; for similar reports on choline, histidine and threonine, we refer to YoungReference Young17.
Findings and mechanisms in different mammals
Tryptophan
A diet high in tryptophan has been shown to reduce mouse killing by ratsReference Gibbons, Barr, Bridger and Leibowitz18, Reference Kantak, Hegstrand, Whitman and Eichelman19, reduce aggression in vervet monkeysReference Chamberlain, Ervin, Pihl and Young20, enhance exploratory behaviour in female silver foxesReference Rouvinen, Archbold, Laffin and Harri21 and reduce self-injurious behaviour in rhesus monkeysReference Weld, Mench, Woodward, Bolesta, Suomi and Higley22. In contrast to the observed reductions in aggression in some experimental conditions, dietary supplementation of tryptophan has also been shown to increase territorial aggression in male miceReference Lasley and Thurmond23. Dietary tryptophan may also influence the resistance or tolerance to stress and, therefore, change the behavioural stress response. Koopmans et al. Reference Koopmans, Ruis, Dekker, van Diepen, Korte and Mroz24 reported enhanced recovery after social stress as measured by lower plasma cortisol and noradrenaline concentrations in pigs fed a surplus of dietary tryptophan compared with pigs fed diets containing a ‘normal’ concentration of tryptophan. In addition, supplementation of dietary tryptophan reduced plasma cortisol concentrations during a stress-inducing mental arithmetic task in healthy stress-vulnerable humansReference Markus, Olivier, Panhuysen, Van Der Gugten, Alles, Tuiten, Westenberg, Fekkes, Koppeschaar and de Haan25. It was, therefore, suggested by Markus et al. Reference Markus, Olivier, Panhuysen, Van Der Gugten, Alles, Tuiten, Westenberg, Fekkes, Koppeschaar and de Haan25 that tryptophan supplementation above normal dietary concentrations could improve the ability of an individual to cope with stress. The effects of dietary tryptophan on stress resistance involve different pathways. In rats a variety of stressors, such as immobilisation, foot shock, and hypothermia, increase brain tryptophan and serotonin turnoverReference Morgan, Rudeen and Pfeil26–Reference Dunn29. Depressed humans show decreased plasma tryptophan concentrations in comparison with normal subjectsReference Branchey, Branchey, Shaw and Lieber30. It appears that initially stressors stimulate serotonin turnover, which over time may deplete serotonin (precursor) supplies and result in decreased serotonin (precursor) concentrations.
Quantitatively the most important pathway for tryptophan metabolism, after protein synthesis, is the kynurenine pathway which is responsible for over 90 % of tryptophan catabolismReference Sainio, Pulkki and Young31. In humans, normally 1 % of the available tryptophan is converted to serotonin which is mainly present in the gastrointestinal tractReference Rodwell, Harper, Rodwell and Mayes32. The first and rate-limiting step in the synthesis of serotonin is the hydroxylation of tryptophan to 5-hydroxytryptophan by the enzyme tryptophan hydroxylase (Fig. 1). Tryptophan hydroxylase is normally about half saturated with tryptophanReference Carlsson and Lindqvist33. Consequently, an increase in tryptophan in the brain, which increases serotonin synthesis and serotonergic neurotransmissionReference Fernstrom and Wurtman34, can maximally double serotonin synthesis. The second step in the synthesis of serotonin is the decarboxylation of 5-hydroxytryptophan to serotonin which is stored in vesicles in the nerve terminal were it is held before release. When serotonin is released into the synaptic cleft, serotonin can bind to different subtype receptors (for reviews, see Barnes & SharpReference Barnes and Sharp35 and Hoyer et al. Reference Hoyer, Hannon and Martin36). Via binding to these different receptors, serotonin can produce many different effects on post-synaptic cells influencing various parts of the brain involved in controlling a variety of physiological functions including hormone releases, cardiovascular functioning, pain, appetite, and in general mood and behaviourReference Barnes and Sharp35–Reference Lucki37.
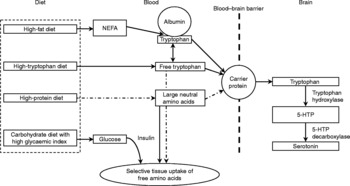
Fig. 1 Effects of dietary characteristics on tryptophan uptake by the central nervous system and synthesis of serotonin from brain tryptophan (adapted from Grimmett & SillenceReference Bourreau, Hernot, Bailhache, Weber, Ferchaud, Biourge, Martin, Dumon and Nguyen185 with modifications). (![]() ), Factors that may ultimately decrease brain tryptophan; 5-HTP, 5-hydroxytryptophan; NEFA, non-esterified fatty acids.
), Factors that may ultimately decrease brain tryptophan; 5-HTP, 5-hydroxytryptophan; NEFA, non-esterified fatty acids.
Tryptophan transport across the blood–brain barrier and metabolism is in part affected by animal factors such as breedReference Bagshaw, Ralston and Fisher38, sexReference Rouvinen, Archbold, Laffin and Harri21, Reference Henry, Seve, Mounier and Ganier39, social statusReference Raleigh, Brammer, McGuire and Yuwiler40, Reference Mench and Shea-Moore41, ageReference Henry, Seve, Mounier and Ganier39, Reference Peremans, Audenaert and Blanckaert42, activityReference Chaouloff, Laude, Guezennec and Elghozi43 and level of arousalReference Trulson and Jacobs44. The availability of dietary tryptophan to the brain is largely dependent on the composition of the ingested diet. Tryptophan is found in nearly all protein-containing foods where it is found in a lower concentration compared with the other large neutral amino acids (LNAA) tyrosine, phenylalanine, leucine, isoleucine and valineReference Spring, Chiodo and Bowen45. For access into the brain, tryptophan shares the same carrier as other LNAA for transport across the blood–brain barrierReference Fernstrom and Wurtman34. Central tryptophan concentrations can either be increased by increasing plasma tryptophan or by lowering plasma concentrations of LNAAReference Fernstrom and Wurtman34, Reference Yuwiler, Oldendorf, Geller and Braun46. As tryptophan is normally present in only small concentrations in dietary protein compared with other LNAA, the consumption of a meal high in protein will decrease the ratio of tryptophan to other LNAAReference Leathwood47 and thereby potentially lower serotonin synthesis.
The fraction of unbound tryptophan as compared with that bound to albumin is another factor that may influence tryptophan availability to the brainReference Chaouloff48. In mammals, approximately 80–90 % of all tryptophan molecules in the blood are bound to serum albuminReference Fuller and Roush49. It has been suggested that the majority of the albumin-bound tryptophan is available for passage across the blood–brain barrierReference Yuwiler, Oldendorf, Geller and Braun46, Reference Pardridge50, but possibly the concentration of circulating free tryptophan may be especially importantReference Chaouloff48. According to ChaouloffReference Chaouloff48, three factors affect circulating free and bound tryptophan concentrations: (i) the rate of lipolysis because blood non-esterified fatty acids displace tryptophan from its binding to albuminReference McMenamy51; (ii) the activity of tryptophan 2,3-dioxygenase, the rate-limiting enzyme in tryptophan detoxication through the kynurenine pathway – activation (inactivation) of this enzyme decreases (increases) circulating blood tryptophan levelsReference Badawy52; (iii) uptake into peripheral and central tissues. Carbohydrate-induced insulin rises facilitate the uptake of most LNAA into skeletal muscle, but not tryptophan bound to albuminReference Pozefsky, Felig, Tobin, Soeldner and Cahill53, Reference Fernstrom and Wurtman54. Consequently, the ratio of tryptophan relative to LNAA increases. This results in a competitive advantage of tryptophan over LNAA for uptake at the blood–brain barrier. However, as little as 2–4 % of the energy of a meal as protein seems to prevent this increased availability of tryptophanReference Sainio, Pulkki and Young31, Reference Benton and Donohoe55.
Tyrosine
In rats, a high-tyrosine diet prevents adverse behavioural and neurochemical effects (for example, immobility during a swim test, depletion of brain noradrenaline) of various acute stressors including hypothermiaReference Rauch and Lieberman56, restraint and tail-shockReference Lehnert, Reinstein, Strowbridge and Wurtman57–Reference Reinstein, Lehnert and Wurtman59. Human studies also suggest beneficial effects of tyrosine under conditions of stress (for reviews, see LiebermanReference Lieberman and Marriott60 and YoungReference Young17).
Tyrosine, which can be synthesised from phenylalanine, is the direct precursor for the catecholamines dopamine, noradrenaline and adrenalineReference Rodwell, Harper, Rodwell and Mayes32. Dopamine can be synthesised from tyrosine in neurons in two steps. The first and rate-limiting step is the conversion of tyrosine to dihydroxyphenylalanine by the enzyme tyrosine hydroxylase. In rats, central tyrosine hydroxylase is approximately 75 % saturated with tyrosineReference Carlsson and Lindqvist33. In the second step, dihydroxyphenylalanine is decarboxylated to dopamine which can be used as an endproduct (neurotransmitter) in neurons or further converted to noradrenaline or adrenalineReference Fernstrom and Fernstrom61. Like tryptophan, tyrosine competes with other LNAA at the blood–brain barrier for entry into the brainReference Fernstrom and Wurtman34 and is taken up into skeletal muscle under the influence of insulinReference Pozefsky, Felig, Tobin, Soeldner and Cahill53, Reference Fernstrom and Wurtman54. In diets, tyrosine is typically available in much higher concentrations compared with tryptophan and high-protein meals will typically raise tyrosine concentrations in the brain, but will lower the concentration of tryptophanReference Wurtman, Hefti and Melamed62. Catecholamines play a key role in a variety of behavioural, neuroendocrine and cardiovascular responses during stressReference Lieberman and Marriott60. Increases in brain tyrosine have little or no effect on catecholamine synthesisReference Young17, but the situation may be different during stress when brain noradrenaline turnover increases and noradrenaline concentrations decreaseReference Lehnert, Reinstein, Strowbridge and Wurtman57, Reference Brady, Brown and Thurmond63. An enhanced noradrenergic activity is part of a normal adaptive stress responseReference Yeghiayan, Luo, Shukitt-Hale and Lieberman64. In stressed rats (tail-shock), ingestion of a high-tyrosine diet reversed the post-stress decline in brain noradrenaline and attenuated behaviour changes, i.e. decreased locomotion, standing on hind legs, hole-poking in a novel open fieldReference Lehnert, Reinstein, Strowbridge and Wurtman57. This suggests that a high-tyrosine diet may be beneficial during severe stress, as it prevents depletion of the substrate required for catecholamine synthesis in times of high catecholaminergic activity and demand.
Findings in dogs
Studies on the effects of tryptophan or tyrosine on behaviour in dogs seem to be limited to one. DeNapoli et al. Reference DeNapoli, Dodman, Shuster, Rand and Gross65 formulated diets with high or low protein content (approximately 310 or 190 g crude protein/kg, respectively) and with or without tryptophan supplementation (1·45 g/kg) in order to provide varying tryptophan contents and tryptophan:LNAA ratios (Table 1). Each of the four diets was fed in random order for 1 week to thirty-three privately owned dogs that displayed a high territorial aggression, dominance aggression or hyperactivity. There was no effect of dietary protein or tryptophan content on the behavioural scores within each group of problem behaviour. However, when the groups of dogs were analysed as one study population a lower territorial aggression score was obtained for dogs fed the high-tryptophan diet compared with dogs fed the low-tryptophan diet, but only when fed a low-protein diet. In addition, dogs fed the high-protein diet without tryptophan supplementation showed a higher dominance aggression score compared with dogs on the other dietary treatments.
Table 1 Effect of dietary protein and tryptophan (TRP) content on canine behaviour

LNAA, large neutral amino acids (tyrosine, phenylalanine, leucine, isoleucine, valine).
* Values are presented on a DM basis.
Three studies in literature have reported that low-protein diets decreased aggression in dogs, though these were not performed under controlled experimental conditions. In a study with seven aggressive golden retrievers held at in-home living conditions, incidences of aggression as reported by their owners immediately decreased after the introduction of a low-protein diet (15–18 % of total energy)Reference Mugford66. Unfortunately, neither the composition of the experimental diet nor the composition(s) of the diet(s) before the dietary intervention were reported. The reduction in aggressive incidences, however, was only sustained in three dogs; two dogs deteriorated again in their behaviour and contact was lost with the remaining two clients. In another study, twelve dogs that exhibited either high territorial aggression, dominance aggression or hyperactivity and fourteen control dogs were fed each of three diets varying in protein content (180, 250 and 310 g crude protein/kg DM) for 2 weeks at in-home living situationsReference Dodman, Reisner, Shuster, Rand, Luescher, Robinson and Houpt67. The low-protein diet and medium-protein diet decreased territorial aggression scores compared with the high-protein diet. No effects of dietary protein content in dogs with dominance aggression or hyperactivity were found. Additional behavioural analysis of the group of dogs demonstrating territorial aggression revealed that five of these dogs showed dominance-related territorial aggression, whereas the other seven dogs showed fear-related territorial aggression. In the latter dogs, territorial aggression decreased when fed the low-protein diet.
For adult dogs fed at maintenance, the minimal dietary tryptophan requirements are currently set at 0·0669 g/1000 kJ (0·28 g/1000 kcal) metabolisable energy (ME) with a tryptophan:LNAA ratio of 0·061 : 1 and for tyrosine and phenylalanine the minimal dietary requirements are 0·3537 g/1000 kJ (1·48 g/1000 kcal) ME68. The Association of American Feed Control Officials (AAFCO)69 has minimum dietary requirements for these nutrients which are slightly higher (0·1099 and 0·4995 g/1000 kJ (0·46 and 2·09 g/1000 kcal) ME, respectively) in order to account for the lower digestibility and availability of nutrients in commercial canine foods compared with semi-synthetic diets. Nutritional guidelines for humansReference Gesch, Hammond, Hampson, Eves and Crowder12 and dogs rarely take behaviour into account as a response criterion, something which has been criticisedReference Lieberman and Committee of Military Nutrition Research and Institute of Medicine70, Reference Reeds71. The minimum quantity of tryptophan in a commercial canine dry expanded diet that has passed a maintenance AAFCO feeding protocol has been reported to be 0·0502 g/1000 kJ (0·21 g/1000 kcal) ME68. The criteria for passing an AAFCO maintenance feeding protocol however, do not take into account animal behaviour. It is unknown if the minimal amount of tryptophan in typical dog foods meets the requirements of the wide variety of dogs, for example, from emotionally stable to anxious individuals, under different conditions, for example, from stress-free to stressful. Both excessive intake and a deficiency of tryptophan are detrimental to the health of an animalReference Sainio, Pulkki and Young31 and are likely to affect behaviour. In horses, a dose of 0·1 mg/kg body weight appears to be too low, causing mild excitationReference Bagshaw, Ralston and Fisher38. In humans, the most common side effect of overfeeding precursors of neurotransmitters has been reported to be nauseaReference Young17. There are currently no requirement estimates for the maximum amount of tryptophan in canine food and it remains to be determined how high-tryptophan diets affect the health of dogs and their behaviour in the long term.
Effects of dietary lipids on behaviour
Lipids have various functions, such as constituents of cellular membranes, precursors for chemical messengers (for example, steroid hormones) and their use as an energy source or stored in the body as adipose tissue. After adipose tissue, the central nervous system has the greatest concentration of lipidsReference Carrié, Clement, de Javel, Francès and Bourre72. The structural constituents in the grey matter of the brain and retinal tissues in mammals are derived from dietary linoleic acid (18 : 2n-6) and α-linolenic acid (18 : 3n-3). Both are polyunsaturated fatty acids (PUFA) and can be metabolised to long-chain PUFA by sequential alternating enzymic desaturation and elongation. Linoleic acid can be metabolised to arachidonic acid (20 : 4n-6) which can be further metabolised to docosapentaenoic acid (22 : 5n-6). The enzymic desaturation and elongation of α-linolenic acid yields eicosapentaenoic acid (EPA) (20 : 5n-3) which can be further metabolised to docosahexaenoic acid (DHA) (22 : 6n-3)Reference Lauritzen, Hansen, Jørgensen and Michaelsen73.
Findings and mechanisms in different mammals
There is ample scientific literature available in which the effects of both dietary deficiency and supplementation of PUFA on animal performance in cognitive or behavioural tests are evaluated (for reviews, see WainwrightReference Wainwright74 and McCann & AmesReference McCann and Ames75). For example, the learning ability of rodents decreased when fed n-3 fatty-acid-deficient dietsReference Bourre, Francois, Youyou, Dumont, Piciotti, Pascal and Durand76, Reference Moriguchi, Greiner and Salem77 and increased when fed DHA-supplemented dietsReference Lim and Suzuki78 compared with rodents fed diets adequate in n-3 fatty acid concentrations. Other studies, however, did not find affects of dietary n-3 PUFA manipulation on learning performance as tested with a Morris water-maze in ratsReference Wainwright, Xing, Ward, Huang, Bobik, Auestad and Montalto79 or miceReference Wainwright, Xing, Mutsaers, McCutcheon and Kyle80. Dietary PUFA seem to affect animal cognition but can also cause behavioural changes. Rats fed n-3 PUFA-deficient diets showed increased aggression scores in a resident intruder testReference DeMar, Ma, Bell, Igarashi, Greenstein and Rapoport81 and increased expression of stress-related behaviours during several stress testsReference Takeuchi, Iwanaga and Harada82 compared with male rats fed adequate amounts of n-3 PUFA. Similarly, anxiety was found to be increased in mice fed a diet deficient in n-3 PUFAReference Carrié, Clement, de Javel, Francès and Bourre83, though others did not observe any effects of dietary PUFA on anxiety in miceReference Francès, Coudereau, Sandouk, Clément, Monier and Bourre84 or ratsReference Chalon, Delion-Vancassel, Belzung, Guilloteau, Leguisquet, Besnard and Durand85.
The dopaminergic and serotonergic systems in the brain are known to play important roles in learning, emotions, and impulse controlReference Lucki37, Reference McEntee and Crook86–Reference Schultz90, which makes it tempting to assume that the effects of PUFA on behaviour run through these systems. Indeed, both systems are known to be influenced by PUFA. Rats deficient in n-3 PUFA compared with rats fed diets with α-linolenic acid showed a reduction in dopamine concentration in the frontal cortexReference Delion, Chalon, Herault, Guilloteau, Besnard and Durand91–Reference Zimmer, Vancassel, Cantagrel, Breton, Delamanche, Guilloteau, Durand and Chalon94 and an increase in dopamine concentration in the nucleus accumbensReference Zimmer, Delion-Vancassel, Durand, Guilloteau, Bodard, Besnard and Chalon93 but no effects in the striatumReference Delion, Chalon, Herault, Guilloteau, Besnard and Durand91, Reference Delion, Chalon, Guilloteau, Besnard and Durand92. In the frontal cortex of these animals the rate of dopamine synthesis and breakdown mediated by monoamine oxidase was not affectedReference Delion, Chalon, Guilloteau, Besnard and Durand92, Reference Zimmer, Vancassel, Cantagrel, Breton, Delamanche, Guilloteau, Durand and Chalon94 and the reduced concentrations may have been linked to the reduced dopaminergic storage poolsReference Zimmer, Vancassel, Cantagrel, Breton, Delamanche, Guilloteau, Durand and Chalon94, Reference Zimmer, Delpal, Guilloteau, Aioun, Durand and Chalon95. Changes in dopamine concentrations were followed by changes in number of D2 receptorsReference Zimmer, Vancassel, Cantagrel, Breton, Delamanche, Guilloteau, Durand and Chalon94. n-3 PUFA-deficient rats had a lower number of D2 receptors in the frontal cortexReference Delion, Chalon, Herault, Guilloteau, Besnard and Durand91, Reference Delion, Chalon, Guilloteau, Besnard and Durand92, Reference Zimmer, Vancassel, Cantagrel, Breton, Delamanche, Guilloteau, Durand and Chalon94 but higher in the nucleus accumbensReference Zimmer, Delion-Vancassel, Durand, Guilloteau, Bodard, Besnard and Chalon93, Reference Zimmer, Vancassel, Cantagrel, Breton, Delamanche, Guilloteau, Durand and Chalon94. Rats fed diets supplemented with EPA and DHA had an increased dopamine concentration and D2 binding possibly as a result of a reduction in monoamine oxidase activity in the frontal cortex compared with rats fed adequate amounts of PUFAReference Chalon, Delion-Vancassel, Belzung, Guilloteau, Leguisquet, Besnard and Durand85.
As for dopamine concentrations, frontal cortex serotonin concentrations were increased in rats fed diets supplemented with n-3 PUFAReference Chalon, Delion-Vancassel, Belzung, Guilloteau, Leguisquet, Besnard and Durand85. In line with this, serotonin in the frontal cortex was reduced in piglets fed n-3 and n-6 PUFA-deficient formula for 18 d from birth compared with piglets fed formula supplemented with linoleic acid and α-linolenic acid and/or arachidonic acid and DHAReference de la Presa Owens and Innis96. The findings in the frontal cortex may not extrapolate to other brain areas. For example, in the hippocampus of 2-month-old rats fed an n-3 PUFA-deficient diet extracellular basal serotonin concentrations were increasedReference Kodas, Galineau, Bodard, Vancassel, Guilloteau, Besnard and Chalon97. This was probably due to reduced storage poolsReference Kodas, Galineau, Bodard, Vancassel, Guilloteau, Besnard and Chalon97, not due to decreased activity of monoamine oxidaseReference Delion, Chalon, Guilloteau, Lejeune, Besnard and Durand98. Such effects of n-3 PUFA deficiency on serotonin concentrations are not found in all studies (for example, Delion et al. Reference Delion, Chalon, Herault, Guilloteau, Besnard and Durand91, Reference Delion, Chalon, Guilloteau, Besnard and Durand92).
In addition to the observed changes in the dopaminergic and serotonergic systems in different brain regions, physical properties (for example, fluidity, permeability) of cerebral membranes may also mediate dietary effects on cognition and behaviourReference Kitajka, Sinclair, Weisinger, Weisinger, Mathai, Jayasooriya, Halver and Puskás99. For example, chronic dietary deficiency in n-3 PUFA resulted in low concentrations of n-3 PUFA in the rat brainReference Bourre, Francois, Youyou, Dumont, Piciotti, Pascal and Durand76, Reference Moriguchi, Greiner and Salem77 whereas diets high in EPA and DHA resulted in high concentrations of EPA and DHA in the brain of ratsReference Chalon, Delion-Vancassel, Belzung, Guilloteau, Leguisquet, Besnard and Durand85, Reference Bourre, Bonneil, Dumont, Piciotti, Nalbone and Lafont100, Reference Bourre, Bonneil, Dumont, Piciotti, Calaf, Portugal, Nalbone and Lafont101. In addition, dietary α-linolenic acid deficiency induces a more pronounced reduction in DHA concentrations in the frontal cortex than in the striatum and cerebellumReference Carrié, Clement, de Javel, Francès and Bourre72, Reference Delion, Chalon, Herault, Guilloteau, Besnard and Durand91. Besides changes in brain PUFA compositions, dietary PUFA may alter properties of the neuronal membrane, such as the activity of membrane-bound enzymes, receptors and ion channelsReference Yehuda, Rabinovitz and Mostofsky102. These alterations may affect neurological functioning and may, therefore, also contribute to the observed changes in cognitive functioning and behaviour.
Findings in dogs
To the authors' knowledge, there are at this moment no scientific articles available regarding the influence of n-3 or n-6 PUFA deficiency or enrichment on canine behaviour or cognitive performance. Since DHA is essential for the development and function of the brain and retinaReference Lauritzen, Hansen, Jørgensen and Michaelsen73, its supply may affect neurological development in puppies. For example, low dietary concentrations of DHA during the gestation or lactation of bitches and dry diets for puppies depressed their retinal sensitivityReference Heinemann, Waldron, Bigley, Lees and Bauer103, Reference Bauer, Heinemann, Lees and Waldron104. Although the immediate connection between the cellular effects of DHA and visual sharpness and cognitive abilities in receiving dietary DHA still needs more supportReference Lauritzen, Hansen, Jørgensen and Michaelsen73, studies seem to emphasise the importance of DHA in the diet of bitches during gestation until weaning and the diet of puppies in order to ensure optimal neurological development. At present, there is no recommended allowance for DHA for both bitches in gestation and lactation or puppies, but the recommended allowance for α-linoleic acid is 3·35 g/1000 kJ (0·8 g/1000 kcal) ME68. A diet high in α-linolenic acid fed from breeding throughout lactation increased α-linolenic acid concentration in milk but failed to do this for DHAReference Bauer, Heinemann, Bigley, Lees and Waldron105, Reference Bauer, Heinemann, Lees and Waldron106. In a recent study, puppies converted α-linolenic acid to DHA during the first month of weaning but little conversion of α-linolenic acid to DHA occurs after weaningReference Bauer, Heinemann, Lees and Waldron106. It seems that the capacity of puppies to synthesise DHA from dietary α-linolenic acid or other n-3 fatty acid precursors is active for only a short time during the neonatal period and is decreased thereafter. The amount of dietary α-linolenic acid for sufficient synthesis of DHA and the amount of DHA required for optimal neurological development in puppies still remain to be determined. Whether the provision of sufficient DHA for optimal neurological development in dogs also results in changes in the dopaminergic and serotonergic systems and subsequent effects in cognitive abilities or behaviour in later life remains to be confirmed.
Concerning commercial dog food, it seems likely that in dogs deficiencies of PUFA are rare as long as fat oxidation during process and storage of the food is limitedReference Biagi, Mordenti, Cocchi and Mordenti107. Levels of PUFA, particularly the n-3 family, are nowadays higher in commercial dog food compared to foods of several years agoReference Delton-Vandenbroucke, Maude, Chen, Aguirre, Acland and Anderson108 (Delton-Vandenbroucke et al., 1998). However, the amount and ratio between n-6 and n-3 fatty acids may differ considerably between commercially available diets. The n-6:n-3 fatty acid ratio of twelve commercial dry dog foods was found to differ considerably, ranging from 17:1 to 5:1Reference Ahlstrøm, Krogdahl, Vhile and Skrede109.
Effects of dietary carbohydrates on behaviour
Feeding of mammals is a discontinuous process in which periods of food consumption are interspersed with periods of non-eatingReference Blundell110. Food intake behaviours are controlled by feelings of hungerReference Rowland, Morien and Li111 and satietyReference Blundell110, but may be modulated by psychological and social factorsReference Read112. Numerous central and peripheral signal molecules are involved in the regulation of eating (for reviews, see BrayReference Bray113, de Graaf et al. Reference de Graaf, Blom, Smeets, Stafleu and Hendriks114 and Strader & WoodsReference Strader and Woods115). The rate and site of degradation of nutrients largely determines the postprandial physiological state of an animal and in this way the extent and duration of satiety and, therefore, behaviour. There is a wide variety of carbohydrates with different physical and chemical properties. These properties can affect the rate and site of degradation of these carbohydratesReference Cummings, Roberfroid, Andersson, Barth, Ferro-Luzzi, Ghoos, Gibney, Hermansen, James, Korver, Lairon, Pascal and Voragen116. In single-stomached animals, degradable carbohydrates may be digested with endogenous enzymes in the first part of the gastrointestinal tract, or fermented by micro-organisms that colonise predominantly the last part of the gastrointestinal tract. Products derived from digestible carbohydrates are mainly monosaccharides. The digestion of starch and absorption of monosaccharides are primarily responsible for the fluctuations in the postprandial blood glucose concentrations that subsequently may modify tryptophan availability in the brain when protein intake is low (see section on Findings and mechanisms in different mammals: Tryptophan), and influence mood in at least humans (for a review, see BentonReference Benton117). The indigestible carbohydrates are often referred to as dietary fibre, which contains non-starch polysaccharides, resistant starch and non-digestible oligosaccharides. The fermentation endproducts of dietary fibre are volatile fatty acids (VFA; acetic, propionic and butyric acid), lactate, alcohol and the gases methane, hydrogen and carbon dioxideReference Bergman118. Apart from the fermentability, other physical and chemical properties of dietary fibre include solubility, ability to bind water and affect viscosity, and possible interactions with the digestion and absorption of starch, protein and fat. In addition, the duration of satiety experienced by animals between meals may be affected by carbohydrates, which in turn may reduce the behavioural side effects of a high feed motivation.
Findings and mechanisms in different mammals
The effects of dietary carbohydrate sources (i.e. fibrous ingredients) on animal behaviour have been relatively well studied especially in pigs, where non-lactating sows were fed energy-restricted diets in order to prevent excessive lipid deposition and reduced reproduction performance. Commonly diets for sows are formulated to meet the daily nutrient requirements for maintenance and reproduction. However, the latter may not result in a sufficient level of satiety between meals and is believed to be an important reason for a persistent high feeding motivation throughout the day contributing to the development of stereotyped behaviourReference Lawrence and Terlouw119. In order to reduce stereotyped behaviour in sows, diets high in fibrous ingredients (sugarbeet pulp, oat hulls, soyabean hulls, wheat bran) can be fedReference Ramonet, Meunier-Salaün and Dourmad120, Reference Bergeron, Bolduc, Ramonet, Meunier-Salaün and Robert121, resulting in an increased time of sows laying downReference Robert, Matte, Farmer, Girard and Martineau122, increased resting time, less time spent on foraging and aggressionReference Danielsen and Verstergaard123 and reduced posture changes 8 and 10 h after feedingReference de Leeuw, Jongbloed and Verstegen124. The latter authors compared sows fed a high- and a low-fermentable carbohydrate diet (for further examples, see Meunier-Salaün et al. Reference Meunier-Salaün, Edwards and Robert125). The relationship between dietary fibre content and stereotyped behaviour has also been documented in horses. A large survey among trainers of race horses in Sweden revealed a negative correlation between the amount of roughage provided and the incidence of stereotyped behaviour (cribbing or wind-sucking, weaving, box-walking) or wood-chewing in horsesReference Redbo, Redbo-Torstensson, Ödberg, Hedendahl and Holm126. Wood-chewing may be related to a ‘fibre deficiency’ in the diet and represent attempts to increase dietary fibre intakeReference Redbo, Redbo-Torstensson, Ödberg, Hedendahl and Holm126–Reference Krzak, Gonyou and Lawrence128. The effect of fibrous ingredients on behaviour is not generic for all fibre sources; for example, solvent-extracted coconut meal and soyabean hulls as a dietary fibre source do not appear to affect physical activity in pigsReference Rijnen, Verstegen, Heetkamp and Schrama129, whereas sugarbeet pulp silage doesReference Rijnen, Verstegen, Heetkamp, Haaksma and Schrama130. Since sows which are fed low amounts of feed were shown to be more active compared with sows fed large amounts of feedReference Spoolder, Burbidge, Edwards, Simmins and Lawrence131 it has been suggested that hunger is most likely the cause of the increased physical activityReference de Leeuw, Zonderland, Altena, Spoolder, Jongbloed and Verstegen132.
The variety in physical and chemical properties of different fibrous ingredients results in differences between these fibres in creating and maintaining satiety and preventing feelings of hunger. The biological mechanisms behind the satiating properties of dietary fibre are still not fully understood, but several dietary fibre characteristics seem to be important. First, fibres with a high water-binding capacity may increase the volume and weight of the gastric contents when liquids are available. The weight or volume may stimulate stretch receptors that can induce gastric signals of satiationReference de Graaf, Blom, Smeets, Stafleu and Hendriks114, Reference van Leeuwen, van Gelder, de Leeuw and van der Klis133. Second, gastric emptying can be affected either directly by dietary fibres high in intragastric viscosityReference Guerin, Ramonet, LeCloarec, Meunier-Salaün, Bourguet and Malbert134 or indirectly through the stimulation of the release of glucagon-like peptide-1 (GLP-1) (a potent inhibitor of gastric emptyingReference Anvari, Paterson, Daniel and McDonald135). Stimulation of GLP-1 production can be mediated through carbohydrate fermentation in the distal part of the gastrointestinal trackReference Cani, Dewever and Delzenne136 or through the production of VFA (mainly acetate) which stimulates the release of peptide tyrosine tyrosine (PYY)Reference Brown, Goldsworthy, Barnes, Eilert, Tcheang, Daniels, Muir, Wigglesworth, Kinghorn, Fraser, Pike, Strum, Steplewski, Murdock, Holder, Marshall, Szekeres, Wilson, Ignar, Foord, Wise and Dowell137–Reference Karaki, Mitsui, Hayashi, Kato, Sugiya, Iwanaga, Furness and Kuwahara139. The effects of GLP-1 and PYY in delaying gastric emptying are referred to as the ‘ileal brake’ mechanism which results in a moderate and stable flow of nutrients from the stomach into the small intestineReference de Graaf, Blom, Smeets, Stafleu and Hendriks114. A decrease in postprandial gastric-emptying rate will, consequently, prolong gastric distension and gastric signals of satiationReference Brown, Goldsworthy, Barnes, Eilert, Tcheang, Daniels, Muir, Wigglesworth, Kinghorn, Fraser, Pike, Strum, Steplewski, Murdock, Holder, Marshall, Szekeres, Wilson, Ignar, Foord, Wise and Dowell137–Reference Karaki, Mitsui, Hayashi, Kato, Sugiya, Iwanaga, Furness and Kuwahara139. This mechanism was studied by Moran et al. Reference Moran, Smedh, Kinzig, Scott, Knipp and Ladenheim141 in rhesus monkeys where intramuscular injections of PYY reduced gastric emptying and resulted in a decrease in food intake. In addition, there are indications that PYY in the brain reduces appetite in humansReference Batterham, Cowley, Small, Herzog, Cohen, Dakin, Wren, Brynes, Low, Ghatei, Cone and Bloom142, although this is still a subject for debateReference Woods143. Third, fibrous dietary ingredients may increase small-intestinal transit timeReference Bueno, Praddaude, Fioramonti and Ruckebusch144, possibly also by stimulation of PYY which is found to suppress intestinal motilityReference Woods143. An increase in small-intestinal transit time: (i) prolongs contact between nutrients and intestinal receptors involved in maintaining satietyReference Houpt146, Reference Lin, Zhao, Chu, Lin and Wang147 and postpones feelings of hungerReference Read112; (ii) results in the slowing down of starch digestion and subsequent absorption of glucose, thereby maintaining more stable postprandial glucose and insulin concentrations in the bloodReference Roberfroid148. A transient decline in blood glucose level preceded meal initiation in ratsReference Louis-Sylvestre and Le Magnen149 and humansReference Campfield, Brandon and Smith150, Reference Campfield, Smith, Rosenbaum and Hirsch151 and caused a delay in the decrease in blood glucose concentrations. This may prolong satiety and postpone hunger and meal initiation (for a review, see Campfield & SmithReference Campfield and Smith152). Finally, fermentation of carbohydrates may yield VFA which leads to a higher level of satiety by (i) PYY-mediated reduction of gastric emptying rateReference Cherbut153 and (ii) becoming a source of energy (mainly acetate) at times when glucose supply from the small intestine is decreasing, which stimulates longer-term satietyReference Bergman118, Reference van Leeuwen, van Gelder, de Leeuw and van der Klis133, Reference Bleiberg, Beers, Persson and Miles154, Reference Rérat155.
As suggested previously, hunger is most likely the cause for the observed behavioural effects seen in sowsReference de Leeuw, Zonderland, Altena, Spoolder, Jongbloed and Verstegen132. Hunger or appetite is correlated with the peripheral concentration of ghrelinReference Wren, Seal, Cohen, Brynes, Frost, Murphy, Dhillo, Ghatei and Bloom156, a twenty-eight amino acid peptide synthesised predominantly in the stomachReference Kojima, Hosoda, Date, Nakazato, Matsuo and Kangawa157, Reference van der Lely, Tschöp, Heiman and Ghigo158. For example, a rise in blood ghrelin concentration is associated with meal initiation in humansReference Cummings, Purnell, Frayo, Schmidova, Wisse and Weigle159. Supplementation of short-chain oligofructose (average degree of polymerization of 4·5) in a diet for 3 weeks decreased energy intake and lowered ghrelin concentrations in rats compared with rats fed the control diet without fructan supplementation. However, rats fed a diet supplemented with long-chain oligofructose (average degree of polymerization of 25·0) showed a decrease in energy intake but not in ghrelin concentrations compared to rats fed the control dietReference Cani, Dewever and Delzenne136. It is suggested that the lower blood ghrelin concentrations may contribute to a decrease in appetite during fastingReference Delzenne, Cani, Daubioul and Neyrinck160. Whether these results were accompanied with changes in behaviour (for example, food-seeking behaviour) requires further investigation. Fig. 2 shows the effects of dietary fibre on satiety.
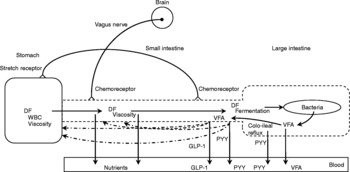
Fig. 2 Effects of dietary fibre (DF) on satiety. (![]() ), Factors that may ultimately increase the residence time of digesta in the designated segments of the gastrointestinal tract; WBC, water-binding capacity; VFA, volatile fatty acids; GLP-1, glucagon-like peptide-1; PYY, peptide tyrosine tyrosine.
), Factors that may ultimately increase the residence time of digesta in the designated segments of the gastrointestinal tract; WBC, water-binding capacity; VFA, volatile fatty acids; GLP-1, glucagon-like peptide-1; PYY, peptide tyrosine tyrosine.
Findings in dogs
‘When we are considering how a dog is behaving, we really should be considering what is inside the stomach’ (MugfordReference Mugford66, p. 1046). Despite this statement, little additional research has been conducted on the association between canine behaviours and satiety or feeding motivation between meals. To the authors' knowledge, three studies have investigated the effects of dietary fibre on satiety and feeding motivation in dogs of which only one also studied canine behaviour and another measured ad libitum food intake of dogs fed diets varying in fibre source and content (Table 2). Butterwick & MarkwellReference Butterwick and Markwell161 fed overweight dogs (>115 % ideal body weight) six different moist diets varying in type and amount of fibre on an energy-restricted basis (45 % restriction of calculated maintenance energy requirements; ME (kJ) = 461 × body weight (kg)0.75). The four experimental high-fibre diets formulated to vary in soluble fibre (SF) and insoluble fibre (ISF), i.e. (g/kg DM) 40·8 SF and 13·6 ISF; 112·5 SF and 37·5 ISF; 35·7 SF and 202·4 ISF; 24·8 SF and 310·6 ISF, were compared with two dry control diets (36·5 SF and 14·6 ISF; 45·5 SF and 15·2 ISF). The authors found no differences in time spent at behaviours related to feeding motivation (i.e. cumulative time spent at feeding bowl and number of visits to bowl 30 min after feeding, intake of a meal (canned diet) provided 3 h after introduction of the test diets) between dogs fed the different diets. In contrast, Jewell & TollReference Jewell and Toll162 did find effects of fibre content on the satiety of dogs. Dogs with ad libitum access to dry diets with a medium or high crude fibre content (135·5 and 223·4 g/kg DM) decreased total ME intake compared with dogs that had ad libitum access to low-crude fibre diets (16·3 and 16·4 g/kg DM). When dogs were offered a subsequent meal, 30 min after the end of the last meal, energy and DM intake were lower in dogs fed the high-fibre diet compared with dogs consuming the low-fibre dietReference Jewell and Toll162. Similarly, Jackson et al. Reference Jackson, Laflamme and Owens163 observed that a high-fibre content in dry diets reduced energy intakes in dogs. These authors fed dogs in the morning either a diet high in total dietary fibre (26·7 SF, 263·7 ISF g/kg as fed) or low in total dietary fibre (18·1 SF, 123·2 ISF g/kg as fed) followed 6 h later by ad libitum access to a diet containing 23·2 SF, 123·5 ISF g/kg as fed. Average energy intake over the day was lower (kJ/kg body weight) in the dogs fed the high-fibre diet in the morning compared with the energy intake of the dogs fed the low-fibre diet in the morning (273 v. 332 kJ (65·3 v. 79·4 kcal)/kg body weight). The difference in average daily energy intake was the result of the energy intake in the morning since there were no significant differences observed in intake of the diet provided in the afternoon between the high-fibre (181 kJ (43·2 kcal)/kg body weight) and low-fibre (197 kJ (47·2 kcal)/kg body weight) groups. These latter two studies showed that high levels of fibrous dietary ingredients in dogs can increase satiety and reduce energy intake. This, however, was not confirmed in a study by Butterwick & MarkwellReference Butterwick and Markwell161. The latter may be due to the energy restriction and the large differences in DM content of diets between studies. Energy restriction will result in an increased feeding motivation in dogs to a level that nullifies the possible effects of fibre on satietyReference Jackson, Laflamme and Owens163. DM content of the moist diets fed to dogs in the study of Butterwick & MarkwellReference Butterwick and Markwell161 ranged between 132 and 168 g/kg whereas Jewell & TollReference Delzenne, Cani, Daubioul and Neyrinck160 and Jackson et al. Reference Jewell and Toll162 fed dry diets with a DM content between 908 and 923 g/kg. On an energy basis, intake of a diet with a high DM content or high energy density will result in lower weight of the digesta in the stomach compared with a diet with similar nutrient composition but lower DM content. A low dietary DM content will therefore have a higher weight of digesta in the stomach and will stimulate stretch receptors which affect satiety in dogsReference Pappas, Melendez and Debas164. Finally, food intake in g DM/kg body weight was found to be lower in dogs with ad libitum access to a diet with 15 g short chain fructo-oligosaccharides/kg DM compared with dogs with ad libitum access to a diet with 60 g cellulose/kg DMReference Howard, Kerley, Sunvold and Reinhart165. The authors suggested that satiety between diets was altered because of the differences in fermentability of the fibre sources included in the diets. Unfortunately, no measurements were made in this study to elucidate possible mechanisms underlying their observed difference in food intake.
The mechanisms behind the observed effects of dietary fibre on inducing and maintaining satiety in humans and pigs (see previous section) have in part been also observed in dogs. Stimulation of stretch receptors through infusion of liquids or filling a balloon with water placed in the stomach reduced sham feeding in dogs, indicating that stimulation of stretch receptors induces satiety in dogsReference Pappas, Melendez and Debas164. Gastric emptying was reduced in dogs as fibre (for example, psyllium, guar gum) content and viscosity of the meal increasedReference Russell and Bass166, which will prolong gastric distension and gastric signals of satiation. In addition, a study of Bueno et al. Reference Bueno, Praddaude, Fioramonti and Ruckebusch144 in which dogs were fed different fibre sources (wheat bran, cellulose, guar gum), both gastric emptying and intestinal transit time were affected with the effect depending on the fibre source included.
A delay in gastric emptying and thus an increase in intestinal transit time by dietary fibre (alginate) results in more stable blood glucose concentrations as observed by Murray et al. Reference Murray, Patil, Fahey, Merchen, Wolf, Lai and Garleb167. In dogs fed a diet with a high level of fermentable fibres (sugarbeet pulp, gum arabic and fructo-oligosaccharides), intestinal GLP-1 concentrations were found to be increased compared with dogs fed a diet with low-fermentable fibre (cellulose) levelsReference Massimino, McBurney, Field, Thomson, Keelan, Hayek and Sunvold168. GLP-1 slows down gastric emptyingReference Anvari, Paterson, Daniel and McDonald135 and intestinal transitReference Daniel, Anvari, Fox-Threlkeld and McDonald169, which may result in prolonged gastric fill and delayed nutrient digestion and absorption. In dogs, the ‘ileal brake’ mechanism may also result from stimulation of the release of PYY by fatty acids sufficient to delay gastric emptying in dogsReference Pappas, Debas, Chang, Taylor and Peptide171.
As reported above, fermentation of carbohydrates yields VFAReference Bergman118, which may lead to prolongation of satiety by becoming a source of energy (mainly acetate) at times when glucose supply from the small intestine is decreasingReference Bergman118, Reference van Leeuwen, van Gelder, de Leeuw and van der Klis133, Reference Bleiberg, Beers, Persson and Miles154, Reference Rérat155. Although dogs have a relatively small and simple large intestine, dogs are capable of fermenting a significant quantity of dietary non-digestible carbohydratesReference Fahey, Flickinger, Grieshop, Swanson, Kamp, Asp, Miller and Schaafsma172. Moreover, the faecal microflora of dogs were found to give similar in vitro organic matter disappearance results compared with the microflora from humans, pigs and horsesReference Sunvold, Hussein, Fahey, Merchen and Reinhart173. The latter indicates that differences between these species in carbohydrate fermentation capacity are probably dependent on factors other than the microbial population. The extent of fermentation in the gastrointestinal tract in an animal largely depends on the time available for microbial fermentationReference Sunvold, Hussein, Fahey, Merchen and Reinhart173–Reference Williams, Bosch, Houdijk and Van der Camp175. In dogs, a transit time through the total gastrointestinal tract between 20 and 35 h is considered normalReference Diez and Istasse176. The large-intestinal transit of digesta can take up to 90 % of the total gastrointestinal transit timeReference Williams, Bosch, Houdijk and Van der Camp175, Reference Bruce, Guilford, Hedderley and McCauley178, presenting a considerable time for large-intestinal microflora to ferment undigested substrates entering from the ileum. The VFA produced can be used by the hindgut bacteria for protein synthesis, resulting in an increase in microbial mass, or absorbed in the large intestine. The contribution of large-intestinal VFA absorption towards the total energy maintenance requirements of dogs has been reported to be approximately 2–7 %Reference Bugaut179, Reference Stevens and Huma180. However, the latter authors did not to provide information on the way these values were derived. In addition, the effect of production and absorption of acetate as an energy source for body tissues on postprandial satiety remains to be investigated. The work of Pouteau et al. Reference Pouteau, Nguyen, Ballevre and Krempf181, Reference Pouteau, Frenais, Dumon, Noah, Martin and Nguyen182 on a method to evaluate acetate production and metabolism using stable isotopes may be the starting point for further exploration of the importance of carbohydrate fermentation in the gastrointestinal tract and satiety in dogs.
To our knowledge, there is no information available in the scientific literature regarding possible influences of dietary fibre on ghrelin concentrations and behaviour in dogs. However, when dogs are fed one scheduled meal per d, ghrelin concentrations increase before and decrease rapidly after the meal to remain relatively constant throughout the rest of the dayReference Yokoyama, Nakahara, Kojima, Hosoda, Kangawa and Murakami183, which may indicate little potency of ghrelin concentrations to affect canine behaviour throughout the day.
Nowadays, dry extruded diets for dogs may contain 30 % or more carbohydrates of which starch is the major component. Moreover, the non-digestible carbohydrate fraction in diets can also make up a considerable proportionReference Fahey, Flickinger, Grieshop, Swanson, Kamp, Asp, Miller and Schaafsma172. As mentioned previously, fibres differing in physical and chemical properties have diverse physiological responses in animals. Nutrient digestion as well as transit time through the gastrointestinal tract may be influenced by the amount and source of fibre included in canine diets. In the case of a reduction in nutrient digestibility when fibres are included, it is necessary to increase the concentration of some nutrients in order to ensure that the nutrient requirements of the animals are metReference Diez, Hornick, Baldwin, Eenaeme and Istasse184. Future canine research on the behavioural effects of dietary fibre should account for the fact that different breeds may respond differently (in terms of satiety). Gastric emptying rate is inversely related to body weight in dogs of different sizesReference Bourreau, Hernot, Bailhache, Weber, Ferchaud, Biourge, Martin, Dumon and Nguyen185. Moreover, large-breed dogs have a longer large-intestinal transit time and increased apparent total dietary fibre digestibility186, which may increase the production and the use of VFA but may increase gastrointestinal discomfort as a result of enhanced fermentation activity.
The degree of satiety in animals such as pigs has been shown to affect behaviour, including aggressive and stereotyped behaviour. Although likely, it is up till now unknown whether canine behaviour can be affected by degree of satiety and further research is required. Assuming that behaviours in dogs are more favourable during times of satiety than during times of hunger as observed in pigs (for example, aggression), specific dietary fibres through their potential to prolong satiety may assist in preventing unwanted canine behaviours.
Conclusions
The present contribution provides an overview of current knowledge on the influence of dietary macronutrient composition on canine behaviour. It can be concluded that little research has been conducted in this field although research in other species indicates that there is potential to modify behaviour in dogs through nutrition. There is evidence that dietary composition can modulate animal and human behaviour through different mechanisms. Dietary protein may contain the precursors tryptophan and tyrosine for the respective neurotransmitters serotonin and catecholamines. Since bioavailability of both tryptophan and tyrosine in the brain are dependent on the dietary protein content and amino acid composition, dietary composition may have an impact on the behaviour and wellbeing of dogs under specific circumstances (for example, stress). However, before application and extrapolation of the evidence found in mostly rodent laboratory studies into commercial canine diets is undertaken, research is required to identify the optimal and safe dietary inclusion level in combination with behavioural tests to study the magnitude of effects on (problem) canine behaviour. The n-3 PUFA have an important role in the development of the brain, and the supply of essential fatty acids such as DHA could affect aspects of the dopaminergic and serotonergic system and, consequently, cognitive performance and behaviour as observed in rodents. Most canine studies and dietary n-3 PUFA have been mainly focused on the effect of maternal intake of different dietary n-3 PUFA during gestation and lactation on n-3 PUFA in the milk and/or n-3 PUFA intake on retinal function of puppies. It would be of interest to examine the DHA required for optimal neurological development and whether this leads to alterations in cognitive abilities or behaviour later in life of dogs. In the literature, studies have been reported which show that, depending on the physical and chemical properties, certain dietary fibres induce satiation or prolongation of satiety after a meal. However, there have been no studies conducted in which the effect of dietary fibre on physiological satiety parameters, behaviour (for example, activity) and/or feeding motivation were studied in dogs. If dietary fibre has short-term effects that result in prolongation of satiety and a reduction of hunger between meals, it may help to prevent unwanted canine behaviours and also promote long-term weight control.





