Introduction
Most of the classical vitamins such as vitamins A, B1, B2, B3, C, D, etc., were discovered by means of the fact that an inadequacy in their supply led to overt forms of deficiency disease such as blindness, beri-beri, pellagra, scurvy, rickets and so on. Consequently, it was easy to establish those food sources that contained such vitamins, since they relieved or prevented the relevant syndromes(Reference Kraemer, Semba and Eggersdorfer1,Reference Semba2) . It is correspondingly hard, by these means, to detect the presence of a vitamin if it is present in virtually every foodstuff that an individual consumes. Recently, however, l-(+)-ergothioneine, hereafter ergothioneine (ERG), has emerged(Reference Paul and Snyder3–Reference Beelman, Kalaras and John10) as an important nutrient, and indeed possible vitamin(Reference Paul and Snyder3), that has precisely these properties of a very widespread occurrence coupled, commonly, to a functional undersupply.
A related class of nutrient, which has not been demonstrated as necessary or essential for life yet provides health benefits when added at levels greater than a normal diet generally provides, has come to be known as nutraceuticals, a coinage based on an amalgamation of ‘nutrition’ and ‘pharmaceutical’(Reference Cencic and Chingwaru11). Interest in such nutraceuticals, also known as ‘functional foods’, has increased enormously over the last few decades(Reference Cencic and Chingwaru11–Reference Spindler, Mote and Flegal22) as our understanding of the important roles of diet in health has improved. However, the enthusiasm for such products has not always been matched by the extent or quality of the evidence for their efficacy(Reference Sharif, Khalid, Holban and Grumezescu20,Reference Poddar, Kolge and Bezman23–Reference Orr28) .
Since ERG classes as a nutraceutical, it seems timely to bring together the extensive but widespread knowledge of its biology so that it may be made more widely available, and that is the purpose of this review.
Discovery and structure
ERG is a somewhat unusual betaine amino acid. It was discovered by Charles Tanret in 1909 while investigating the ergot fungus Claviceps purpurea (Reference Tanret29,Reference Tanret30) . It is also known as 2-mercaptohistidine trimethylbetaine, and its formal International Union of Pure and Applied Chemistry (IUPAC) name is (2S)-3-(2-thioxo-2,3-dihydro-1H-imidazol-4-yl)-2-(trimethylammonio)propanoate. It is an l-histidine derivative that is Nα,Nα,Nα-trimethyl-l-histidine in which the hydrogen at position 2 on the imidazole ring is replaced by a mercapto group. Its structure(Reference Barger and Ewins31), and those of some related molecules, is given in Fig. 1, indicating that is a tautomer that has both a thiol and a thione form. Although it is a thiol, and hence an antioxidant(Reference Servillo, Castaldo and Casale32,Reference Franzoni, Colognato and Galetta33) , the thione tautomer is predominant at physiological pH(Reference Hand, Taylor and Honek34,Reference Hand and Honek35) , and this makes it unusually resistant to autoxidation, i.e. simple oxidation by molecular O2 (Reference Servillo, Castaldo and Casale32,Reference Fahey36–Reference Fahey38) . Its midpoint potential for a thiol is consequently unusually high, being +0·06 V v. −0·2 to −0·4 V for typical thiols including glutathione(Reference Cheah and Halliwell4,Reference Jacob39–Reference Walz41) and mycothiol(Reference Reyes, Pedre and De Armas42,Reference Sharma, Van Laer and Messens43) , and −0·193 V for the also somewhat oxidising thiol cofactor coenzyme M, which is 2-mercaptoethanesulfonate(Reference Kell and Morris44). Its reaction with hydroxyl radicals (OH•) is virtually instantaneous, while it reacts only more slowly with H2O2 and/or O2 •− (Reference Fahey38). Its Se equivalent is known as selenoneine and also has strong antioxidant properties(Reference Achouba, Dumas and Ouellet45–Reference Yamashita, Yabu and Yamashita52), but is not otherwise discussed here.
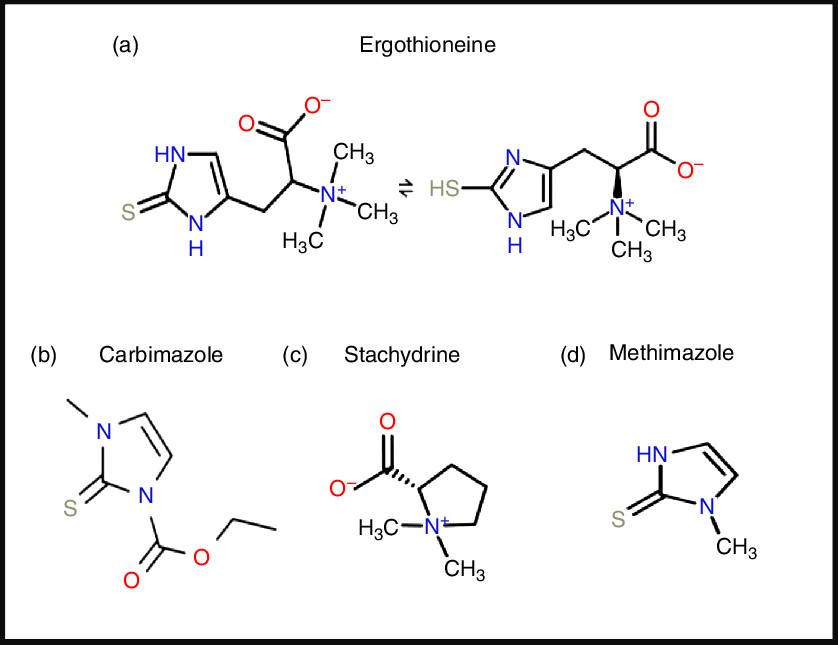
Fig. 1. Structures of ergothioneine and related molecules. For a colour figure, see the online version of the paper.
From a pharmaco-chemical point of view ERG is also unusual, since – using our standard substructure analysis(Reference O’Hagan and Kell53,Reference O’Hagan and Kell54) in KNIME(Reference O’Hagan and Kell55) – we note that just two drugs marketed for human consumption (the anti-thyroxine-production drug methimazole and its pro-drug carbimazole, Fig. 1), and no endogenous genome-encoded metabolites from Recon2(Reference Thiele, Swainston and Fleming56) contain the imidazole-2-thione substructure(Reference O’Hagan, Swainston and Handl57). This said, a good many fungicides do contain the benzimidazole substructure(Reference Lewis, Tzilivakis and Warner58), and a variety of benzothiazoles are used as dyes.
Biosynthesis and phylogenetic distribution
A particular feature of ERG is that although it is more or less universally distributed among higher organisms, none of them – as is consistent with the idea that it may in fact be a vitamin requiring exogeneous sources – can in fact biosynthesise it. The chief organisms capable of its synthesis are fungi and certain yeasts(Reference Melville, Genghof and Inamine59,Reference Jones, Doyle and Fitzpatrick60) , though actinobacteria and certain other micro-organisms(Reference Jones, Doyle and Fitzpatrick60–Reference Trivedi, Singh and Bhat66), including the slime mould Physarum polycephalum (Reference Genghof65), cyanobacteria(Reference Narainsamy, Farci and Braun67–Reference Liao and Seebeck71) and methylotrophs(Reference Alamgir, Masuda and Fujitani72) are also naturally capable of its production. The related mycothiol is typically ten times more concentrated in actinobacteria than is ERG(Reference Rawat and Av-Gay73), and its biosynthetic pathway might provide an antitubercular drug target. Other organisms acquire ERG through transporter-mediated uptake. Thus higher plants contain it but do not biosynthesise it(Reference Melville and Eich74); instead they and other organisms(Reference Baran, Bowen and Price68,Reference Baran, Brodie and Mayberry-Lewis75) take it up from fungal production in the soil(Reference Melville76–Reference Warren79), and possibly via actinobacterial(Reference Park, Lee and Kim80) or fungal(Reference Park, Lee and Kim80,Reference Guo, Lin and Wang81) symbionts. Animals are also considered not to biosynthesise it(Reference Melville, Otken and Kovalenko82,Reference Melville, Horner and Otken83) , and accumulate it using a particular transporter, detailed below, via the plants and animals that they eat. Although not easy, it is possible to raise animals such as pigs on a diet such as casein, sucrose, lard, butter and salts that is considered to lack ERG; such animals are said to have undetectable levels of the compound(Reference Eagles and Vars84), and rats treated similarly have reproduced(Reference Melville, Horner and Lubschez85,Reference Kawano, Otani and Takeyama86) . However, we do not know the minimum amount and its location that animals need, and these are old experiments that need to be repeated with modern techniques with lower detection limits. Only then might we have a definitive statement as to whether ERG is absolutely required as a true vitamin or not, and if so in what amounts for health. In a similar vein, ERG can be present in cell culture media and cells with organic cation transporter N1 (OCTN1)/solute carrier family 22, member 4 (SLC22A4) can accumulate it(Reference Tucker, Cheah and Halliwell87), a fact little considered to date in cell culture studies.
To the extent that ERG is a ‘secondary’ metabolite, defined(Reference Bu’Lock88) as a molecule whose synthesis has a relatively restricted distribution in different organisms, the biosynthetic pathways diverge from primary metabolism via the amino acids histidine, cysteine and methionine(Reference Melville, Eich and Ludwig89–Reference Naowarojna, Cheng and Chen94). Thus (Fig. 2), histidine is trimethylated using S-adenosyl methionine to form trimethyl histidine, also known as hercynine(Reference Reinhold, Ishikawa and Melville95,Reference Melville, Ludwig and Inamine96) . This reacts oxidatively with cysteine to form hercynylcysteine sulfoxide(Reference Ishikawa, Israel and Melville97), which is converted to ERG. In some organisms, hercynine takes a more convoluted route via γ-glutamylhercynylcysteine sulfoxide (Fig. 2)(Reference Naowarojna, Cheng and Chen94). Table 1 provides references for different organisms. An excellent phylogenetic analysis is given by Jones et al. (Reference Jones, Doyle and Fitzpatrick60). In more recent work, it has been suggested that ERG was probably first biosynthesised by anaerobes using a slightly different route that converts hercynine directly to ERG(Reference Leisinger, Burn and Meury98–Reference Ruszczycky and Liu100), and that was later repurposed.
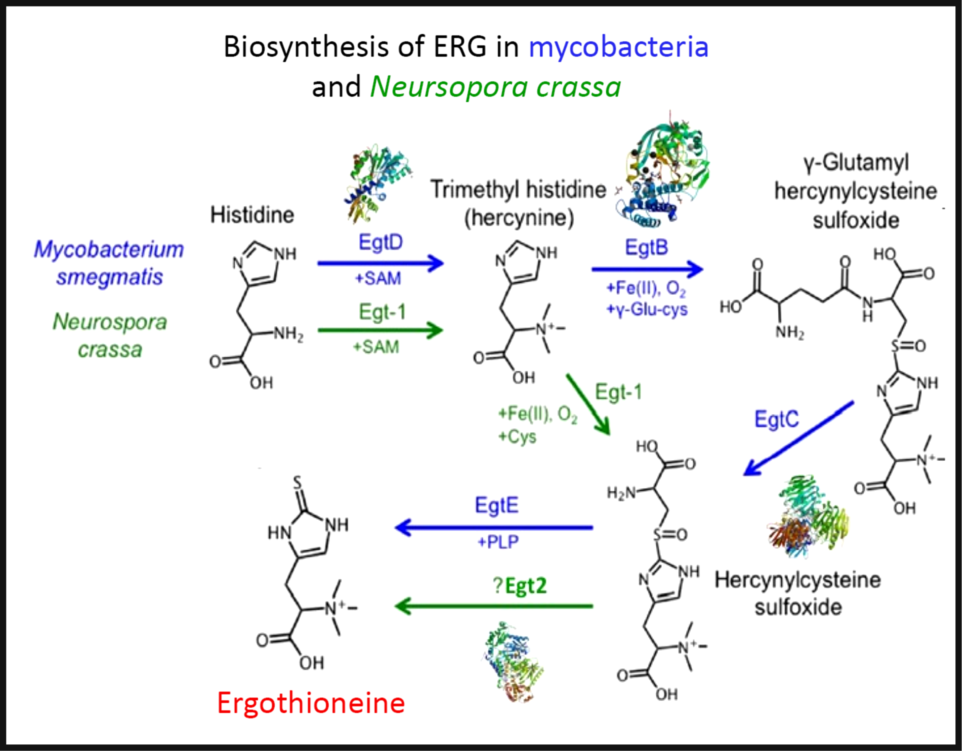
Fig. 2. The two main pathways of aerobic ergothioneine (ERG) biosynthesis, noting the relevant enzymes and thumbnails of three-dimensional structures where known. SAM, S-adenosyl methionine. For a colour figure, see the online version of the paper.
Table 1. Biosynthesis of ergothioneine in various non-recombinant micro-organisms

Three-dimensional structures are known for a number of the relevant enzymes, including mycobacterial EgtB(Reference Goncharenko, Vit and Blankenfeldt101) for example, PDB 4XBE, EgtC(Reference Vit, Mashabela and Blankenfeldt102) for example, PDB 4ZFJ, EgtD(Reference Vit, Misson and Blankenfeldt103–Reference Misson, Burn and Vit105) for example, PDB 4PIM, and Neurospora crassa early G1 transcript 2 (egt2) which is like egtE(Reference Irani, Naowarojna and Tang106) for example, PDB 5UTS. Very recently, EgtB from Candidatus Chloracidobacterium thermophilum was crystallised(Reference Naowarojna, Irani and Hu107), and engineered towards Egt1 activity. Thumbnails are given in Fig. 2. Egt1 from N. crassa is 876 amino acids long(108), while egtD (from Mycobacterium tuberculosis (109)) is just 321 amino acids long; since the N-terminal sequences are well conserved (Fig. 3), this implies an extra C-terminal domain catalysing the production of hercynylcysteine sulfoxide from hercynine.

Fig. 3. Alignment of Neurospora crassa Egt1 and N-terminal part of Mycobacterium tuberculosis EgtD. For a colour figure, see the online version of the paper.
In addition, enantiopure l-ERG has been synthesised chemically(Reference Melville76,Reference Daunay, Lebel and Farescour110–Reference Khonde and Jardine112) , and by fermentation of genetically engineered micro-organisms (Table 2). Initial efforts in ERG synthesis were carried out in Schizosaccahromyces pombe using egt1 overexpression under an inducible promoter. The N starvation and glucose starvation conditions causing long quiescence led to the maximum ERG production of 1606·3 µm while the wild-type strain produced 0·3 µm (Reference Pluskal, Ueno and Yanagida50). Methylobacterium aquaticum strain 22A was engineered by expressing an additional copy of egtBD genes and by deleting the gene encoding histidine ammonia lyase, which degrades an ERG precursor l-histidine. The resulting strain produced up to 7·0 mg EGT/g dry cell weight and 100 μg EGT/5 ml per 7 d in test-tubes(Reference Fujitani, Alamgir and Tani113). The filamentous fungus Aspergillus oryzae has also been engineered to produce ERG by expression of egt1 and egt2 genes from N. crassa, resulting in 231 mg ERG per kg of solid media(Reference Takusagawa, Satoh and Ohtsu114).
Table 2. Fermentative production of ergothioneine in recombinant micro-organisms
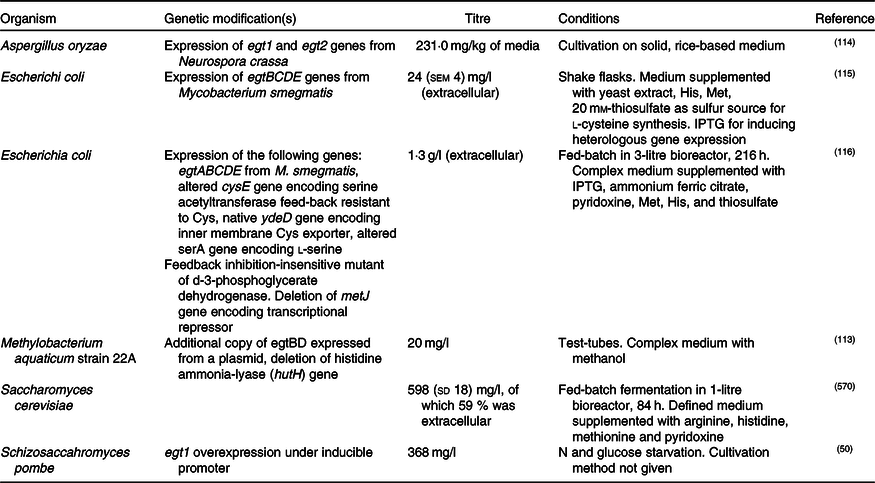
IPTG, isopropyl β- d-1-thiogalactopyranoside.
Expression of egtBCDE genes from Mycobacterium smegmatis in Escherichia coli and optimisation of medium composition has led to 24 mg/l or 104 μm of secreted ERG(Reference Osawa, Kamide and Satoh115). The egtA gene from M. smegmatis was not expressed because E. coli contains a homologous glutamate–cysteine ligase encoded by gshA and involved in glutathione biosynthesis. In a follow-up study, the authors expressed egtA from M. smegmatis and it had a positive effect on ERG production. Furthermore, they enhanced cysteine and S-adenosine methionine biosynthesis and obtained 1·3 g/l or ERG in a fed-batch fermentation(Reference Tanaka, Kawano and Satoh116), achieving currently the highest titre reported for heterologous ERG production.
Recently, we reported the engineering of baker’s yeast Saccharomyces cerevisiae for the production of ERG(Reference van der Hoek, Darbani and Zugaj117). S. cerevisiae has a generally recognised as safe (GRAS) status and has been exploited for the commercial production of several nutraceutical compounds(Reference Li and Borodina118); it is thus a highly attractive host for the production of ERG. We have tested sixteen different pathway variants, nine containing only fungal genes, one with bacterial genes from M. smegmatis, and six hybrid pathway variants containing both fungal and bacterial transgenes. The best-performing strain contained egt1 from N. crassa and egt2 from C. purpurea. The composition of the medium was improved using a fractional factorial design. Fed-batch cultivation resulted in 598 (sd 18) mg/l ERG after an 84-h fermentation. Some 60 % of the measured ERG was extracellular and the rest accumulated in the cells. Table 2 summarises the various recombinant expression hosts that have been used.
The distribution of solute transporters between tissues in differentiated organisms is particularly heterogeneous(Reference O’Hagan, Wright Muelas and Day119), and it is to be expected that both SLC22A4 and ERG might also be distributed heterogeneously as well. This is indeed the case, their distribution being especially high in tissues that are considered to have the potential for oxidative stress(Reference Cheah and Halliwell4), such as erythrocytes(Reference Chaleckis, Ebe and Pluskal120–Reference Mitsuyama and May129), bone marrow(Reference Gründemann, Harlfinger and Golz130), liver and kidney(Reference Melville, Horner and Lubschez85,Reference Tang, Cheah and Yew131) , seminal fluid(Reference Nikodemus, Lazic and Bach132,Reference Kaneko, Takeuchi and Yamaoka133) and the lens and cornea of the eyes(Reference Shires, Brummel and Pulido134). It may also be accumulated in the CNS(Reference Crossland, Mitchell and Woodruff135,Reference Nakamichi, Taguchi and Hosotani136) .
Finally, here, we note – as with the activity of the ‘master Fe regulator’ hepcidin(Reference Vermeulen and Vermeersch137–Reference Reichert, da Cunha and Levy141), that acts chiefly via the ferrous Fe transporter ferroportin – that the action of a transporter in concentrating a substance in one tissue will typically lead to its depletion from another. Consequently, it is necessary to measure all relevant compartments to assess whether a molecule such as ERG, whose distribution is strictly transporter-mediated, is protective against a particular disease/effect or otherwise in a particular place or case.
SLC22A4: the ergothioneine transporter
Although this view remains controversial, even hydrophobic molecules do not normally ‘float across’ whatever phospholipid bilayer portion of cells may be untrammelled by proteins. Xenobiotics in particular need to ‘hitchhike’ on protein transporters that have presumably evolved for ‘natural’ substrates but that are capable of their uptake(Reference Kell, Swainston and Pir142–Reference Dickens, Rädisch and Chiduza152). While transporters seem to have remained somewhat understudied(Reference César-Razquin, Snijder and Frappier-Brinton153), those transporters involved in uptake and encoded by the human genome are now catalogued formally as SLC for solute carriers(Reference Hediger, Clemencon and Burrier154,155) , with efflux transporters mainly being classed as ABC families(Reference Chen, Shi and Zhang156).
One solute carrier, previously known as organic cation transporter N1 (OCTN1)(Reference Koepsell157,Reference Pochini, Galluccio and Scalise158) , and now known as SLC22A4 (the human version is Uniprot Q9H015), a 551-amino-acid transporter with three glycosylation sites, is of special interest. It had been designated as a transporter of carnitine and of the (non-physiological) tetraethylammonium cation. However, in a really groundbreaking paper, Gründemann et al. (Reference Gründemann, Harlfinger and Golz130) recognised that the rates observed (using radioisotopes) were too small to be physiologically meaningful, and using a method that we would now refer to as ‘untargeted metabolomics’(Reference Garg, Kapono and Lim159–Reference Dunn, Erban and Weber164), they incubated two kinds of HEK293 cells in serum. The first were normal cells, that, as with many transporters(Reference O’Hagan, Wright Muelas and Day119), do not in fact express SLC22A4 at significant levels, while the second had been engineered to overexpress the transporter. They then simply looked for those molecules that were most differentially taken up, a molecule called stachydrine, also known as proline betaine, being the main one observed, Stachydrine is a constituent of citrus juices(Reference Heinzmann, Brown and Chan165–Reference Lloyd, Beckmann and Favé167). Some elementary cheminformatics based on structure similarity searches(Reference O’Hagan, Swainston and Handl57,Reference Gasteiger168) indicated that ERG, as a betaine, was indeed similar to stachydrine. Incubating the cells just with ERG showed that it was taken up about 100 times more quickly than was tetraethylammonium, leading to the designation of SLC22A4 as ‘the’ ERG transporter(Reference Gründemann, Harlfinger and Golz130). Subsequent work(Reference Tucker, Cheah and Halliwell87,Reference Bacher, Giersiefer and Bach169–Reference Tschirka, Kreisor and Betz172) has confirmed and reinforced this view of SLC22A4 and its homologues(Reference Shimizu, Masuo and Takahashi173) as having significant specificity for ERG, and weak activity for various drugs(Reference Yabuuchi, Tamai and Nezu174–Reference Tamai177). It is concentrative, coupled in humans to influx of 2 or 3 Na+ ions per ERG transported(Reference Gründemann, Harlfinger and Golz130). Interestingly, it is up-regulated chronobiologically just before meal times(Reference Akamine, Koyanagi and Kusunose175). The rat and human orthologues are interchangeable(Reference Nakamura, Yoshida and Yabuuchi178). Tissue levels of ERG depend on an exogenous supply(Reference Darghouth, Giarratana and Oliveira179), but are then well correlated with the expression levels of SLC22A4(Reference Paul and Snyder3,Reference Taubert, Lazar and Grimberg180) . SLC22A4 expresses well even in microbial systems(Reference Indiveri, Galluccio and Scalise181), and is widely tolerant of amino acid substitutions(Reference Frigeni, Iacobazzi and Yin182). As yet, no other transporter with significant activity for ERG in humans is known, making it a potentially interesting drug target(Reference Nigam183,Reference Pochini, Scalise and Galluccio184) .
Expression patterns
SLC22A4 is known to express in the intestinal lumen(Reference Wolf, Paine and McQueen185), acting to take up ERG, as well as some xenobiotics including pyrilamine, quinidine and verapamil, and having multiple known but weak inhibitors.
Fig. 4 shows the expression of the transcript for SLC22A4 in fifty-six cell lines using previous data(Reference O’Hagan, Wright Muelas and Day119) taken from the human protein atlas(Reference Thul, Åkesson and Wiking186), indicating a range in expression levels between different cell lines of more than 4000-fold, a number not atypical for human transporters(Reference O’Hagan, Wright Muelas and Day119). Tissue expression data are given in Fig. S4 of O’Hagan et al. (Reference O’Hagan, Wright Muelas and Day119).
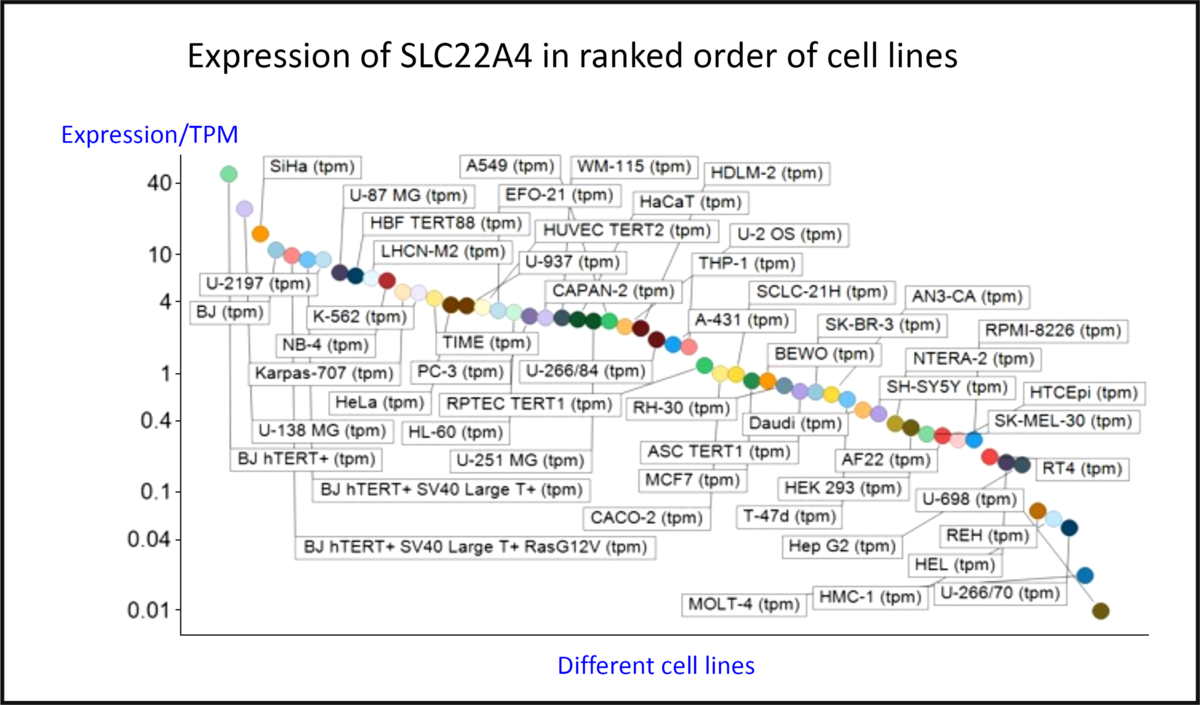
Fig. 4. Differences in expression of SLC22A4 transcript in a series of mammalian cell lines. Data are from Thul et al. (Reference Thul, Åkesson and Wiking186) and O’Hagan et al. (Reference O’Hagan, Wright Muelas and Day119). For a colour figure, see the online version of the paper.
The intracellular expression patterns are as yet uncertain, with early claims for a mitochondrial expression(Reference Kawano, Otani and Takeyama86,Reference Apostolova and Victor187–Reference Xuan, Lamhonwah and Librach190) being based on very weak and contradictory evidence(Reference Kerley, McCarthy and Kell8). However, while the cellular uptake of ERG does require plasma membrane expression, the latest version of the protein atlas indicates mitochondrial expression as well(191). However, as is well known, antibody specificities are rarely either known or absolute(Reference Skogs, Stadler and Schutten192–Reference Michaud, Salcius and Zhou198). Thus, relying on antibody evidence alone is rather hazardous, and, as mentioned before(Reference Kerley, McCarthy and Kell8), mitochondrial transporters have an SLC25 family designation(Reference Palmieri199,Reference Palmieri200) . Definitive measurements on the uptake or otherwise of ERG into isolated mitochondria, or indeed into other organisms that cannot make it, are eagerly awaited.
Evolution and phylogenetic distribution of SLC22A4
Phylogenetic analyses(201,202) indicate that homologues of SLC22A4, a relative of the major facilitator superfamily 2, exist only in vertebrate animals, especially mammals, birds and fish, with occasional examples in reptiles (for example, Xenopus spp.).
In practice, it appears that the transporters responsible for the uptake of some 85 % of pharmaceutical drugs actually evolved to take up exogenous natural products(Reference O’Hagan and Kell203). In the case of the cocaine transporter(Reference Chapy, Smirnova and Andre204), a simple narrative can serve to explain how a cocaine-mediated ability to outrun a predator such as a sabre-tooth tiger can rather obviously be selected provided the bioactive substance is actually taken up by the host. More generally, the ability to transport exogenous natural products is likely to be selected for when these confer fitness benefits on the host(Reference Danchin205), and this probably underpins the finding that successful, marketed drugs are indeed similar to (mainly ‘secondary’) natural products(Reference O’Hagan and Kell203).
Oxidative stress
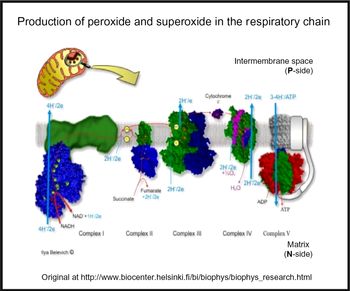
Oxidative stress is widespread to the point of ubiquity in chronic, inflammatory diseases(Reference Kell and Pretorius206,Reference Butterfield and Halliwell207) , with over fifty papers having the words ‘oxidative’, ‘stress’ and ‘review’ in their titles at PubMed in 2018 alone! It can occur when oxygen tension is low and respiratory chains are over-reduced such that they reduce O2 with one electron to superoxide or two electrons to H2O2, instead of the four that are used during the reduction of dioxygen to water by cytochrome oxidase(Reference Kell208) (Fig. 5). Peroxides are also produced in vivo by various oxidases and peroxidases, such as xanthine oxidase, by reduction of dioxygen (for example, Babior(Reference Babior209), Cave et al. (Reference Cave, Brewer and Narayanapanicker210) and Bedard & Krause(Reference Bedard and Krause211)).
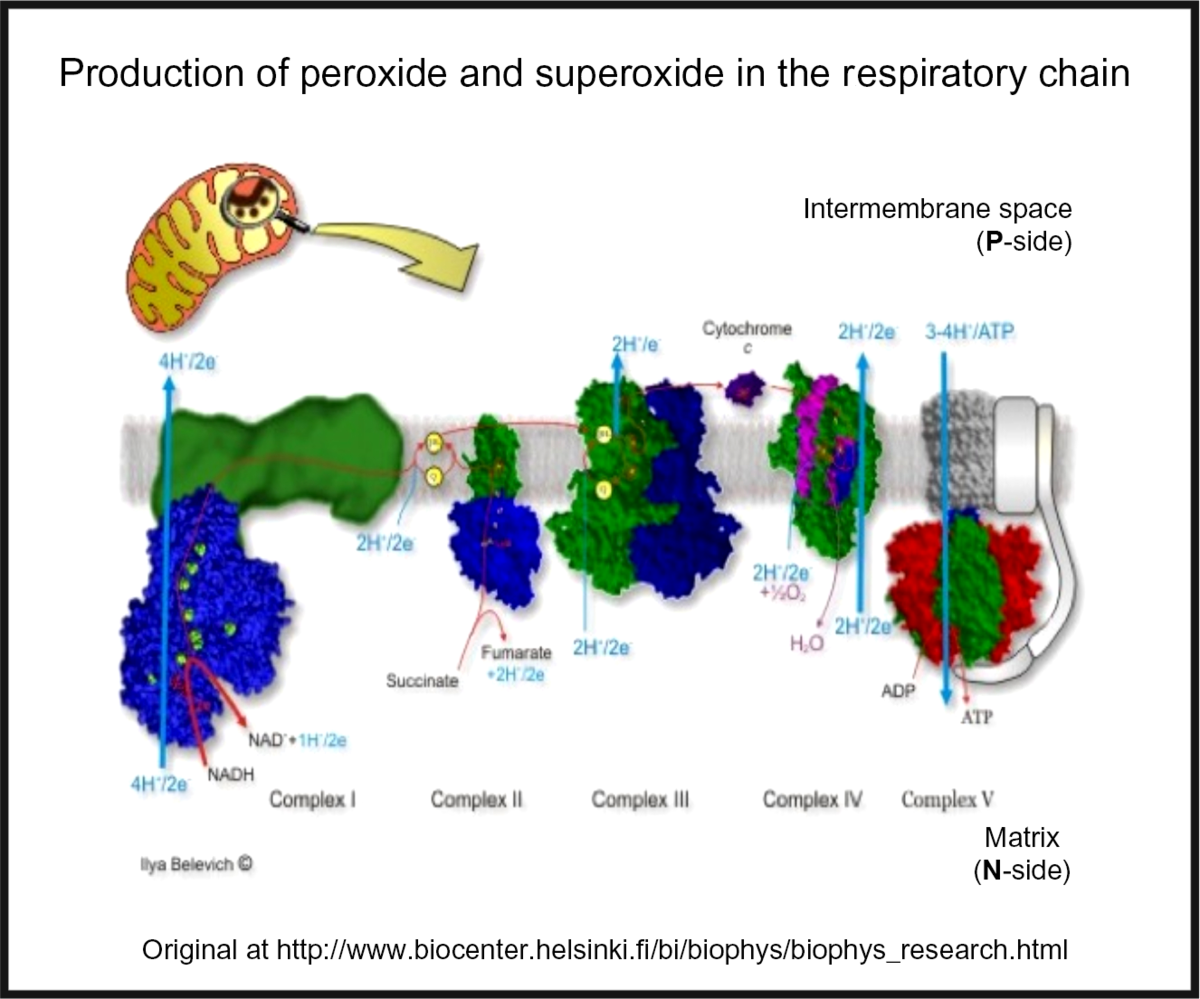
Fig. 5. Superoxide and peroxide are produced by 1- and 2-electron reduction of dioxygen by the mammalian respiratory chain. For a colour figure, see the online version of the paper.
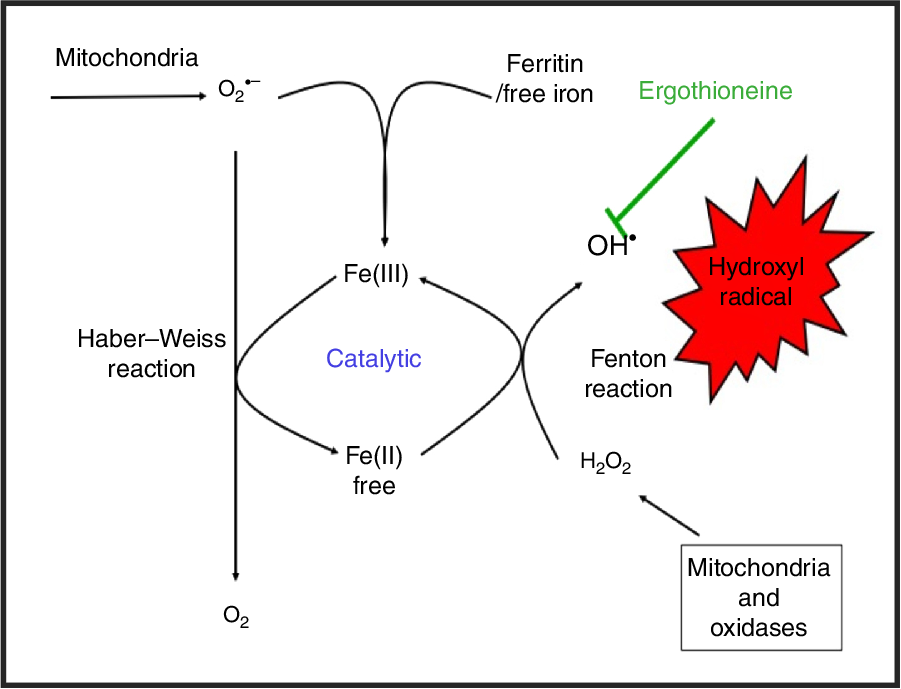
While H2O2 and superoxide are certainly capable of effecting unwanted oxidations, it is the hydroxyl radical that is the key. Thus an important reaction of H2O2 with (free or poorly liganded) Fe(II) is the Fenton reaction(Reference Kell208,Reference Wardman and Candeias212,Reference Kell213) , leading to the very reactive and damaging hydroxyl radical (OH•):
which can react within nanoseconds with anything adjacent. The role of Fe is absolutely vital here(Reference Kell208,Reference Kell213) . Superoxide can also react with ferric Fe in the Haber–Weiss reaction(Reference Winston, Feierman and Cederbaum214–Reference Kehrer216) to produce Fe(II) again, thereby effecting redox cycling, and meaning the ‘iron’ is catalytic (Fig. 6):
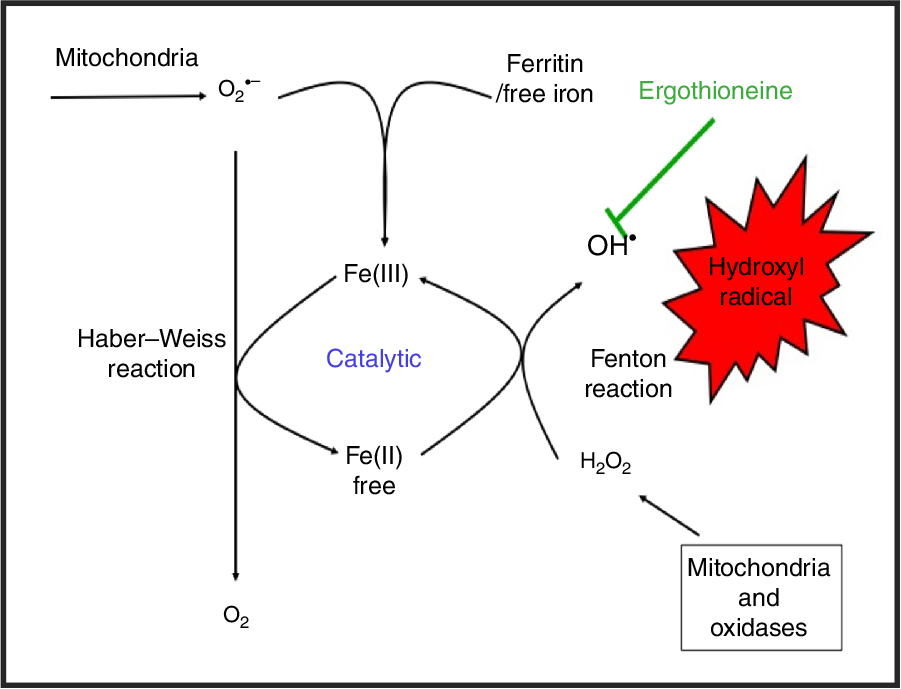
Fig. 6. Catalytic roles of unliganded iron in hydroxyl radical production via the Fenton and Haber–Weiss reactions. This can be stopped by ensuring that iron is fully liganded. For a colour figure, see the online version of the paper.
In addition O2 •− can release ‘catalytic’ Fe from Fe-S clusters in certain proteins and from ferritin(Reference Kell208,Reference Kell and Pretorius217) , another way in which it can promote the Fenton reaction. Note that other reactions can produce OH• anaerobically(Reference Valachová, Mach and Dubovický218). Because OH• is so reactive it is not really observable in its free form; its action is detected via products of molecules with which it has reacted. These include 8-oxo-guanine derivatives(Reference Migliore, Fontana and Colognato219), nitrotyrosine(Reference Ahsan220–Reference Ryberg and Caidahl222) (itself formed from peroxynitrite(Reference Rahman223,Reference Aruoma, Whiteman and England224) , possibly formed more commonly via superoxide(Reference Halliwell225,Reference Ferrer-Sueta, Campolo and Trujillo226) ), 4-hydroxy-nonenal(Reference Petersen and Doorn227), and many others reviewed previously(Reference Kell208). In evaluating the antioxidant potency of ERG or anything else, it is molecules such as these that are normally assessed. Although the literature is somewhat scattered and heterogeneous, it seems clear that as well as hydroxyl radicals(Reference Akanmu, Cecchini and Aruoma228–Reference Chaudière and Ferrari-Iliou232), ERG can also react with and detoxify, or prevent the formation of, singlet oxygen(Reference Oumari, Goldfuss and Stoffels233–Reference Stoffels, Oumari and Perrou242), ozone(Reference He, Krone and Scherl243), superoxide(Reference Jang, Aruoma and Jen231,Reference Obayashi, Kurihara and Okano241,Reference Markova, Karaman-Jurukovska and Dong244–Reference Servillo, D’Onofrio and Casale246) , peroxide(Reference Servillo, Castaldo and Casale32,Reference Li, Yang and Sit124,Reference Bello, Barrera-Perez and Morin247,Reference Hartman248) , hypochlorite(Reference Servillo, Castaldo and Casale32,Reference Chaudière and Ferrari-Iliou232,Reference Asahi, Wu and Shimoda249) and peroxynitrite(Reference Aruoma, Whiteman and England224,Reference Jang, Aruoma and Jen231,Reference Whiteman and Halliwell250,Reference Aruoma, Spencer and Mahmood251) . Consequently, it is a potent antioxidant.
Roles in the producer
Although it is not a priori certain that they would be the same in both producer and consumer organisms, it is of interest, before looking at higher organisms, to consider the roles of ERG in the producer organisms themselves. In the case of C. purpurea, the ERG serves as an antioxidant to neutralise a plant host defence response based on H2O2 that would otherwise inhibit the production of its conidia(Reference Garay252,Reference Garay253) . In M. tuberculosis and other mycobacteria(Reference Sao Emani, Williams and Wiid254), and also in other actinobacteria(Reference Nakajima, Satoh and Yanashima255) and in fungi(Reference Bello, Barrera-Perez and Morin247,Reference Liu, Zhao and Guo256,Reference Sheridan, Lechner and Keeffe257) , it is clear that ERG can have a role as an antioxidant(Reference Trivedi, Singh and Bhat66,Reference Ta, Buchmeier and Newton258–Reference Cumming, Chinta and Reddy260) and also act as a buffer against reductive stress(Reference Farhana, Guidry and Srivastava261). In nature many organisms can be subjected to oxidative stress, and produce a variety of molecules to combat it(Reference Kurutas262–Reference Davey and Kell270). This also seems true of mushrooms(Reference Savoie, Minvielle and Largeteau271,Reference Yokota, Frison and Marcante272) , where ERG is typically the main antioxidant(Reference Kozarski, Klaus and Jakovljevic273–Reference Dubost, Ou and Beelman275), and where it may also inhibit the oxidative enzyme tyrosinase(Reference Liao, Wu and Tsai276). Given suggestions that the ‘purpose’ of secondary metabolite formation is to serve as a signalling molecule in different cells of the producer organism, i.e. as a pheromone(Reference Kell, Kaprelyants and Grafen277), it is interesting to note that this may also involve crosstalk of ERG(Reference Sheridan, Dolan and Doyle37), due in part to the complex networks in which it may be embedded(Reference Gallagher, Owens and Dolan278). The same is true of the imidazole thiol-containing ovothiol(Reference Song, Her and Raso279,Reference Castellano and Seebeck280) . In a similar vein, and although outwith our scope here, we note the potential of other antioxidant natural products such as curcumin(Reference Vázquez-Fresno, Rosana and Sajed281–Reference Tomeh, Hadianamrei and Zhao286).
Nutritional sources
Betaines are generally seen as nutritionally beneficial(Reference Pekkinen, Rosa-Sibakov and Micard287), and many are ‘compatible solutes’(Reference Kempf and Bremer288–Reference Fahnert293), defined as solutes whose accumulation assists the survival of the organism when undergoing various kinds of stress such as osmotic or thermal stress. However, of these, only ERG is seen as a major antioxidant. Although a variety of foodstuffs such as oats(Reference Ey, Schömig and Taubert294,Reference Lee, Alexander and Jonnalgadda295) contain ERG because they take it up from exogenous sources, it is really mushrooms that are the prime sources for humans(Reference Rathore, Prasad and Sharma18,Reference Ey, Schömig and Taubert294) . Indeed, ERG has been proposed as a nutritional biomarker for mushroom consumption(Reference Wang, Gapstur and Carter296,Reference Pallister, Jennings and Mohney297) , albeit that different mushrooms typically contain different amounts(Reference Dubost, Ou and Beelman275,Reference Dubost, Beelman and Peterson298–Reference Kalaras, Richie and Calcagnotto300) , and these can vary with physiological or environmental conditions(Reference Tepwong and Ohshima301–Reference Kalaras, Beelman and Holick305). Those with the highest amounts include oyster mushrooms (Pleurotus spp., up to 4 mg/g DM)(Reference Liang, Ho and Huang306), the golden oyster Pleurotus citrinopileatus with 10·65 mg/g DM(Reference Lin, Chien and Wang307,Reference Lin, Chien and Wang308) , and shiitake (Lentinula edodes, about 1 mg/g DM), while of those more common outside Asia, porcini or ceps (Boletus edulis, > 7 mg/g DM), stand out(Reference Halliwell, Cheah and Tang6,Reference Ey, Schömig and Taubert294,Reference Kalaras, Richie and Calcagnotto300) . However, even common field or ‘button’ mushrooms, Agaricus bisporus, contain some 0·4 mg/g DM(Reference Dubost, Ou and Beelman275,Reference Dubost, Beelman and Royse299,Reference Kalaras, Richie and Calcagnotto300,Reference Chen, Ho and Hsieh309) . Note too that tempe(h), the result of a solid substrate Rhizopus oligosporus fermentation(Reference Peñaloza, Davey and Hedger310–Reference Nout and Kiers314), also contains high levels of ERG(Reference Halliwell, Cheah and Tang6). Mushrooms may also be a significant benefit to those seeking a meat-free diet as they can be made to share certain organoleptic features with meat(Reference Guinard, Myrdal Miller and Mills315,Reference Myrdal Miller, Mills and Wong316) . Notably, ‘the production of cultivated, edible mushrooms worldwide has increased more than 30-fold since 1978, whereas the population has only increased by about 1·7-fold during the same period’(Reference Beelman, Kalaras and John10,Reference Royse, Baars, Tan, Zied and Pardo-Giménez317) .
Some studies that have demonstrated nutritional/health benefits of mushrooms and their antioxidant activity(Reference Weigand-Heller, Kris-Etherton and Beelman125,Reference Savoie, Minvielle and Largeteau271,Reference Gallego, Rojas and Falcón318–Reference Zembron-Lacny, Gajewski and Naczk351) ) did not always seek to deconstruct these into their constituents such as ERG, but ERG is clearly the chief mushroom antioxidant. We note too that some effects may be dependent on the composition of the gut microflora(Reference Jayachandran, Xiao and Xu352), that are of course themselves likely to be changed by ERG, just as they are by many other non-antibiotic drugs(Reference Maier, Pruteanu and Kuhn353).
Safety
Producer organisms such as mushrooms are well known to make many secondary metabolites, some of which can be highly toxic(Reference Schmutz, Carron and Yersin354–Reference Verma, Bhalla and Kumar356) and by various mechanisms(Reference White, Weinstein and De Haro357). Notwithstanding the highly variable intake between individuals(Reference Ramirez-Martinez, Wesolek and Yadan358), however, a number of high-dose studies have indicated that ERG is safe for mammalian consumption at levels far in excess of those likely to be encountered in foodstuffs(Reference Weigand-Heller, Kris-Etherton and Beelman125,Reference Tang, Cheah and Yew131,Reference Marone, Trampota and Weisman359,Reference Cheah, Tang and Yew360) , and it has been declared safe by relevant committees such as those of the European Food Standards Agency(Reference Turck, Bresson and Burlingame361,Reference Turck, Bresson and Burlingame362) . It also lacks any detectable mutagenicity or genotoxicity in such assays, even at very high doses(Reference Schauss, Vértesi and Endres363,Reference Schauss, Béres and Vértesi364) .
Analytics
Leaving aside early efforts such as the colorimetric methods of Hunter(Reference Hunter365), of Melville and colleagues(Reference Melville76,Reference Melville, Horner and Lubschez85,Reference Melville and Lubschez366) and of Carlsson et al. (Reference Carlsson, Kierstan and Brocklehurst367), a variety of analytical methods have been proposed(Reference Cheah and Halliwell4), mostly involving capillary electrophoresis(Reference Sotgia, Arru and Sotgiu368,Reference Sotgia, Pisanu and Pintus369) or chromatography(Reference Sotgia, Arru and Sotgiu368,Reference Zhou, Liu and Jiang370–Reference Sotgia, Zinellu and Arru372) coupled to absorbance(Reference Liu, Zhang and Wang373,Reference Muda, Pelizzoni and Sello374) , fluorescence detection(Reference Newton, Dorian and Fahey375–Reference Sotgia, Pisanu and Cambedda378), electrochemical detection(Reference Kuninori and Nishiyama379) or MS(Reference Alamgir, Masuda and Fujitani72,Reference Wang, Thuya and Toh127,Reference Liu, Zhao and Guo256,Reference Sotgia, Arru and Sotgiu368,Reference Sotgia, Pisanu and Cambedda378,Reference Kroepfl, Francesconi and Schwerdtle380–Reference Sotgia, Zinellu and Pintus382) . A useful feature is that ERG is unusually stable, in that anhydrous ERG decomposes only at 275–276°C(Reference Melville76), allowing its isolation at temperatures close to that of boiling water(Reference Alamgir, Masuda and Fujitani72). As judged by the reversibility of its acid–base titration(Reference Sakurai and Takeshima383), it is also stable to extremes of pH.
Industrial purification of glycine betaine is done by extraction with water(Reference Bessieres, Gibon and Lefeuvre384) and subsequent ion exchange chromatography(Reference Bessieres, Gibon and Lefeuvre384,Reference Kar and Singhal385) , which can be done in simulated moving bed fashion(Reference Bubnik, Pour and Gruberova386). Glycine betaine can then be crystallised(Reference Bessieres, Gibon and Lefeuvre384). As glycine betaine is structurally similar to ERG, this straightforward industrial process could potentially be adapted for ERG.
Serum and other concentrations
While most ERG is inside erythrocytes in whole blood(Reference Halliwell, Cheah and Tang6,Reference McMenamy, Lund and Wallach121,Reference McMenamy, Lund and Neville122,Reference Mitsuyama and May129,Reference Kumosani387) , there have been a number of measurements of ERG concentrations in serum. Unsurprisingly it varies with diet(Reference Baldridge and Lewis388,Reference Baldridge389) , starvation(Reference Teruya, Chaleckis and Takada390), age(Reference Cheah, Feng and Tang391,Reference Yan, Wu and Lin392) and other factors, including diseases of oxidative stress(Reference Dang, Shi and Werstuck393), with typical values of 20–100 µg/ml. A detailed list is provided by Cheah & Halliwell(Reference Cheah and Halliwell4); a smaller listing is given in Table 3. Interestingly, ERG is also present in seminal fluid(Reference Leone and Mann394–Reference Strzeżek, Koziorowska-Gilun and Kielczewski396) and human breast milk(Reference Halliwell, Cheah and Tang6). Any possible correlation with male fertility(Reference Kenny and Kell397) seems not to have been established, though there were no negative effects(Reference Forster, Spézia and Papineau398), and ERG improved oocyte quality and maturation in cows and sheep(Reference Mishra, Reddy and Dhali399). ERG is also present in eye lens, where its concentration is lower in individuals with cataracts(Reference Shukla, Kulshrestha and Khuteta400).
Table 3. Concentrations of ergothioneine in human serum

* Molecular weight = 229·3, so 1 mm = 229 mg/l.
Metabolism and excretion
ERG is metabolised and excreted only slowly(Reference Cheah, Tang and Yew360,Reference Mayumi, Kawano and Sakamoto371,Reference Kato, Kubo and Iwata401,Reference Chaleckis, Murakami and Takada402) . In a recent and detailed study, Cheah et al. (Reference Cheah, Tang and Yew360) administered 5–25 mg daily doses of ERG to human volunteers for 7 d. There was little urinary excretion (<4 %), and the main metabolites were hercynine, plus lesser amounts of S-methyl-ERG, whose concentrations were well correlated with the level of ERG and the dose of ERG given. Similar observations were made in mice(Reference Tang, Cheah and Yew131). Various other biomarkers of oxidative stress (for example, 8-iso-PGF2α from lipid peroxidation) were lowered concomitantly in the human study, attesting to the antioxidant functions of ERG in vivo, although in this case the healthy young subjects were probably not suffering from oxidative stress. There was also quite some variation in uptake between individuals, presumably reflecting variation in their expression of SLC22A4. Agrobacterium radiobacter (Reference Jose403) and other bacteria(Reference Kelly and Appleman404–Reference Maurer, Leisinger and Lim409) contain an ergothionase that degrades ERG to thiolurocanic acid (3-(1H-imidazol-5-yl)prop-2-enethioic S-acid) and trimethylamine, also implying that such cells possess one or more transporters for ERG. The thiolurocanic acid can be further degraded to glutamate(Reference Booth and Appleman410).
Apparent fitness benefits and bioactivities of ERG and the role of SLC22A4
Given the great technical difficulties associated, because of its ubiquity, with withholding ergothoneine from a human or animal diet, one means of ‘removing’ ERG from a host is to remove the ERG transporter by genetic means. This has in fact been done in mice(Reference Kato, Kubo and Iwata401); such SCL22A4–/– mice had immeasurably low levels of ERG relative to controls, and were much more sensitive to oxidative stress than were the wild type. Similar effects were observed in Caenorhabditis elegans (Reference Cheah, Ong and Gruber411). Polymorphisms in SLC22A4, of which there can be many(Reference Tamai177,Reference Ben Said, Grati and Ishimoto412–Reference Toh, Cheung and Murray415) , under selection(Reference Mathieson, Lazaridis and Rohland416), have also been associated with ischaemic stroke(Reference Yamase, Horibe and Ueyama417), erythroid differentiation(Reference Nakamura, Sugiura and Kobayashi418), hearing loss(Reference Ben Said, Grati and Ishimoto412), rheumatoid arthritis(Reference Reglinski, Smith and Wilson126,Reference Taubert, Lazar and Grimberg180,Reference Maeda, Hirayama and Kobayashi419–Reference Lee, Bae and Kim427) , lupus(Reference Orozco, Sánchez and Gómez428), Crohn’s disease(Reference Kato, Kubo and Iwata401,Reference Taubert, Grimberg and Jung429–Reference Leung, Hong and Fraser436) , hearing loss(Reference Ben Said, Grati and Ishimoto412), type 1 diabetes(Reference Santiago, Martinez and de la Calle437) and diabetic embryopathy(Reference Zhao and Reece438). The expression of SLC22A4 can itself be modulated by other factors, including by PPAR-α activity(Reference Wada, Koyanagi and Kusunose439). The very diversity of these diseases speaks naturally to a broad and common underlying cause, the easiest of which involves mechanisms of oxidative stress, inflammation and cell death.
Mechanisms of action
It has become common to discover a binding of a small molecule to another molecule such as a protein, and assume that this interaction, leading to activation or inhibition of the target, constitutes ‘the’ mechanism of action of the small molecule at a physiological level. Unfortunately this is rarely the case, and known drugs, despite often being selected for inhibiting potently a specific molecular target(Reference Kell147), have, on average, six known binding targets(Reference Mestres, Gregori-Puigjané and Valverde440). When these interactions ramify through a complex and non-linear biochemical network it can be hard to apportion the effects of a small exogenous molecule between the various interactions(Reference Kell441–Reference Sastry, Gao and Szubin443).
A standard view of systems or network biology (for example, Kell(Reference Kell444) and Kell & Knowles(Reference Kell, Knowles, Szallasi, Stelling and Periwal445)) develops these ideas in four stages. In stages 1 and 2 we simply recognise the actors and the interactions between them at a qualitative level. Stages 3 and 4 then seek to describe the equations reflecting individual steps and the values of the parameters of those equations. Armed with these we can make an ordinary or, if spatial resolution within a compartment is required, a partial differential equation model of the system. This can then be run and the sensitivities of each step determined(Reference Ihekwaba, Broomhead and Grimley446–Reference Rand448). We are very far from this last part, and so studies of the effects of ERG have in general(Reference Kell and Oliver449) been rather descriptive in nature. Many have been at the level of processes rather than mechanisms, and they have been reviewed in detail(Reference Paul and Snyder3,Reference Cheah, Tang and Yew360) . Table 4 and Fig. 7 provide a selection of determinands that have been shown to change their concentrations or activities when ERG is added to the system of interest. In many cases it is not at all clear what the proximate mechanisms are. Note as just one example that the highly promiscuous transcription factor NF-κB(Reference Ledoux and Perkins450–Reference Shih, Wang and Yang452), whose frequency-dependent activity directly affects the expression of hundreds of enzymes(Reference Nelson, Ihekwaba and Elliott453,Reference Ashall, Horton and Nelson454) , is itself redox-sensitive(Reference Buelna-Chontal and Zazueta455–Reference Gao and Dudley458), and is affected by ERG(Reference Xiao, Zhao and Li459,Reference Rahman, Gilmour and Jimenez460) , while NF-κB increases the rates of SC22A4 transcription(Reference Maeda, Hirayama and Kobayashi419). Thus, deconstructing the many possible direct and consequential interactions of ERG with proteins, v. whether these are simply a consequence of its provision of a more reducing environment, is likely to be a formidable task. In a similar vein, the effects of ERG on the microbiomes of the hosts are likely to be significant, but do not yet seem to have been studied.
Table 4. Biological properties whose expression or activity varies on exposure of a biological system to ergothioneine (ERG) or a modulation of SLC22A4 activity
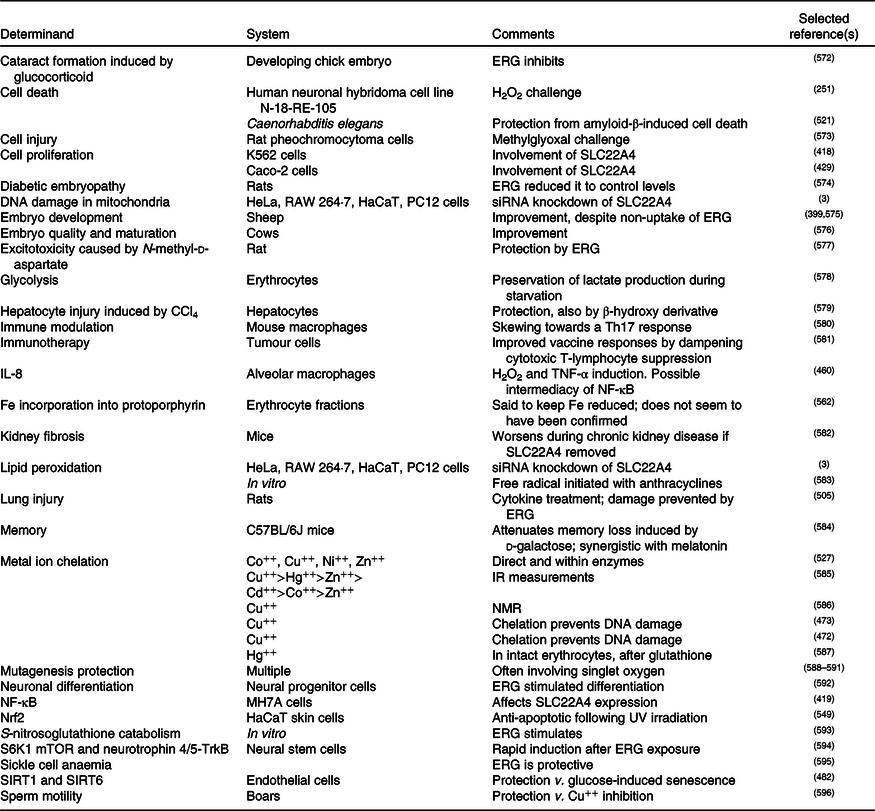
siRNA, small interfering RNA; mTOR, mammalian target of rapamycin; SIRT, sirtuin.
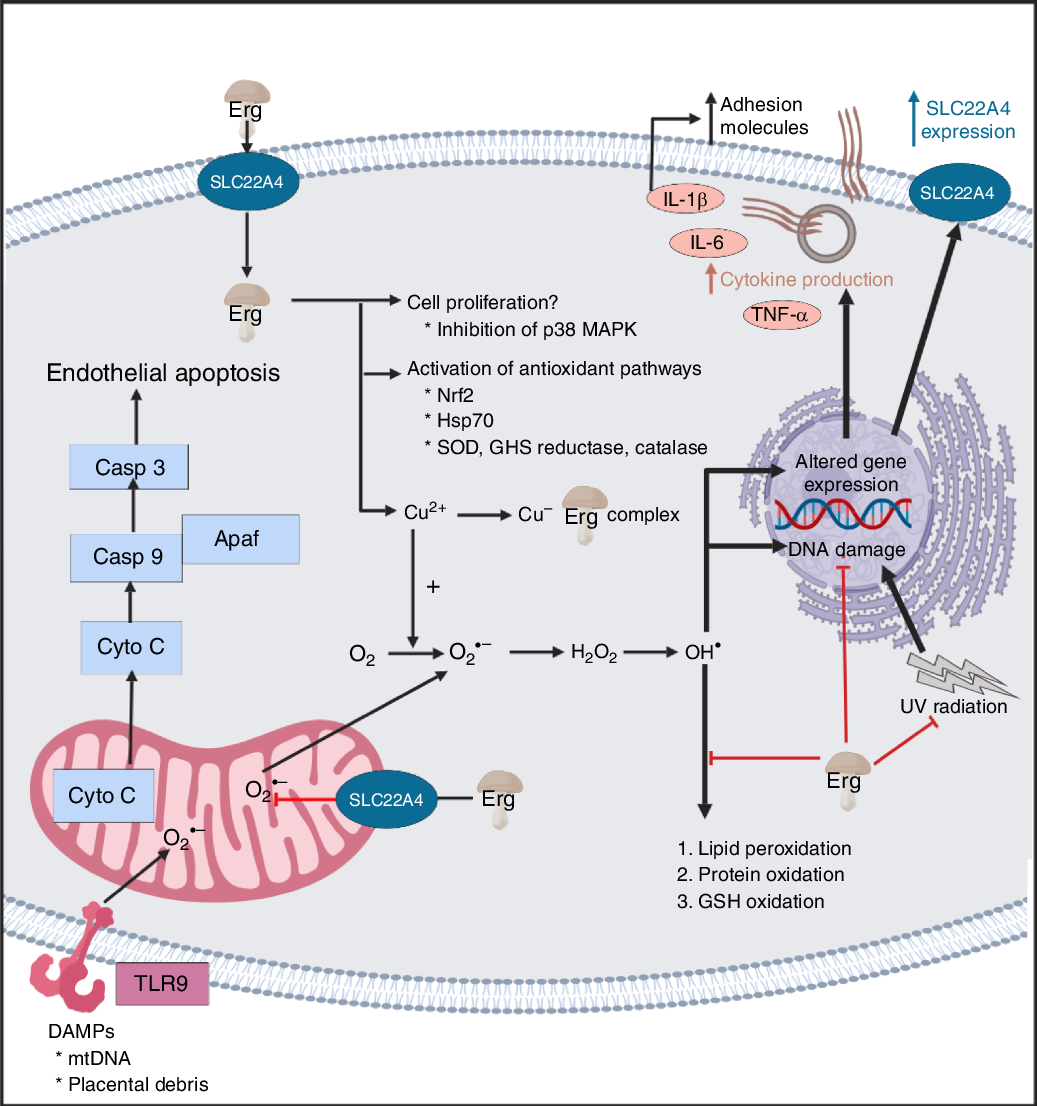
Fig. 7. Overview of some of the effects of ergothioneine in mammalian systems. For a colour figure, see the online version of the paper.
It seems clear that the chief role of ERG, via a variety of mechanisms, including directly, is to serve as an antioxidant and cellular protectant against various kinds of reactive oxygen and N species.
Cytoprotection
At a high level, ERG is seen as an excellent cytoprotectant against all kinds of cellular insults(Reference Paul and Snyder3,Reference Cheah and Halliwell4,Reference Halliwell, Cheah and Tang6,Reference Li, Yang and Sit124) . We split some of the more detailed analyses into subdivisions in the following few sections.
Oxidative stress
Oxidative stress can be defined and measured in many ways(Reference Agarwal, Aponte-Mellado and Premkumar461–Reference Halliwell and Gutteridge468), but is broadly taken to involve a dysregulation in the various redox systems of the organism of interest, coupled to the production of various ‘reactive oxygen species’, principally peroxide, superoxide, hydroxyl radical, and singlet oxygen. ERG has been shown to decrease oxidative stress in the liver and kidney of rats(Reference Deiana, Rosa and Casu469), rescued cells from β-amyloid-induced apoptotic death(Reference Jang, Aruoma and Jen231), protected against palmitic acid-induced cell death(Reference Laurenza, Colognato and Migliore470), mercuric chloride-induced cellular dysfunction(Reference Gökçe, Arun and Ertuna471), and prevented Cu-induced oxidative damage to DNA(Reference Rai, Chalana and Karri472,Reference Zhu, Mao and Fan473) . It is protective against the oxidation of various kinds of molecule(Reference Aruoma, Spencer and Mahmood251,Reference Gokce and Arun474) , including astaxanthin(Reference Pahila, Ishikawa and Ohshima475), and accumulates in a guinea-pig model of non-alcoholic fatty liver disease(Reference Cheah, Tang and Ye476), massively so in mouse models of myocardial infarction and heart failure(Reference Sansbury, DeMartino and Xie477), and in a rat model of optic nerve crush(Reference Agudo-Barriuso, Lahoz and Nadal-Nicolás478). It serves to resist H2O2-induced cell death(Reference Colognato, Laurenza and Fontana479), pyrogallol-induced toxicity(Reference Li, Yang and Sit124), cisplatin-(Reference Song, Chen and Liao480) or oxaliplatin-induced(Reference Nishida, Takeuchi and Hosoda481) toxicity, glucose-induced senescence(Reference Servillo, D’Onofrio and Casale246,Reference D’Onofrio, Servillo and Giovane482) , as well as lipopolysaccharide-induced inflammation(Reference Gunawardena, Bennett and Shanmugam483). In particular, it is protective against ischaemia–reperfusion injury(Reference Sakrak, Kerem and Bedirli484–Reference Arduini, Eddy and Hochstein486), and may have uses in prolonging the lifetime of stored blood(Reference Zimring, Smith and Stowell487). Probably such antioxidant activities are at the core of its biological benefits.
Ergothioneine as a therapeutic for chronic inflammatory diseases?
Inflammation and oxidative stress go hand in hand(Reference Paul and Snyder3), since reactive oxygen species (and materials such as bacterial cell wall components that can lead to their generation(Reference Kell and Pretorius206,Reference Kell and Pretorius488) ), lead to the production of inflammatory cytokines. Although a great many chronic, inflammatory diseases are recognised as having an oxidative stress component(Reference Rahman223), the history of treating them with antioxidants such as ascorbate has largely been a litany of failure, with the treatment arm often even giving worse prognoses than the placebo(Reference Halliwell, Cheah and Tang6,Reference Rahman223,Reference Goodman, Bostick and Kucuk489–Reference Bjelakovic, Nikolova and Gluud501) . Arguably this is because nominally antioxidant molecules such as ascorbate have complex, multi-electron redox chemistry, and can in fact act as pro-oxidants(Reference Mendes-da-Silva, Lopes-de-Morais and Bandim-da-Silva502,Reference Zhang and Omaye503) , especially in the presence of free Fe(Reference Kell208,Reference Kell213) or Cu(Reference Ullah, Khan and Zubair504). This is not an issue with ERG, however, partly because it can chelate them, and ERG levels are decreased, or ERG has been proposed as a useful antioxidant, in diseases such as acute respiratory distress syndrome(Reference Repine and Elkins505), CVD(Reference Martin506,Reference Servillo, D’Onofrio and Balestrieri507) , chronic obstructive pulmonary disease(Reference Rahman223), pre-eclampsia(Reference Kerley, McCarthy and Kell8) (see also Turner et al. (Reference Turner, Brewster and Simpson128)), overhydrated hereditary stomatocytosis(Reference Darghouth, Koehl and Heilier508), and is significantly lowered in others such as certain leukaemias(Reference McMenamy, Lund and Wallach121,Reference McMenamy, Lund and Neville122) . The evidence for this comes from a variety of sources, including metabolite measurements in human subjects(Reference McMenamy, Lund and Wallach121,Reference McMenamy, Lund and Neville122,Reference Smith, Ottosson and Hellstrand509) , and intervention studies in both animals(Reference Repine and Elkins505) and cell lines(Reference Paul and Snyder3,Reference Li, Yang and Sit124,Reference Martin506) . In particular, there is a notable relationship between ERG consumption and longevity (Fig. 6 in Beelman et al. (Reference Beelman, Kalaras and John10)), while in a 3236-participant Swedish study, ERG was the metabolite most strongly connected to a ‘health conscious food pattern’ and was associated with a lower risk of coronary disease (hazard ratio (HR) per 1 sd increment of ERG, HR = 0·85; P = 0·01), cardiovascular mortality (HR = 0·79; P = 0·002) and overall mortality (HR = 0·86; P = 4 × 10–5)(Reference Smith, Ottosson and Hellstrand509).
Neurological diseases and cognitive function
Mushrooms have been shown to have very substantial effects on cognitive function(Reference Mori, Inatomi and Ouchi341,Reference Feng, Cheah and Ng348,Reference Nurk, Refsum and Drevon510–Reference Thangthaeng, Miller and Gomes513) , and this is mainly ascribed to their ERG content, that also deceases with the age of the consumer(Reference Cheah, Feng and Tang391). The kinds of evidence include both double-blind, placebo-controlled clinical trials(Reference Mori, Inatomi and Ouchi341) and observational (cross-sectional) studies in both humans(Reference Feng, Cheah and Ng348,Reference Nurk, Refsum and Drevon510–Reference Phan, David and Sabaratnam512) and rodents(Reference Thangthaeng, Miller and Gomes513). Thus, consuming 1·5 mushroom servings per week was associated with a halving of the incidence of mild cognitive impairment (a precursor of Alzheimer’s dementia), while intake of nine servings per week was associated with a five-fold decrease(Reference Feng, Cheah and Ng348). Note, however, that at least one mushroom trial indicated no measurable benefits in healthy young physical education students(Reference Tsuk, Lev and Rotstein514). Brain and serum ERG levels are also markedly different in Parkinson’s disease(Reference Hatano, Saiki and Okuzumi515), reviewed in Hang et al. (Reference Hang, Basil and Lim516), Shao & Le(Reference Shao and Le517) and Shah & Duda(Reference Shah and Duda518), and even in sudden infant death syndrome(Reference Graham, Chevallier and Kumar519), and ERG has been shown to be protective against β-amyloid-induced neuronal injury(Reference Yang, Lin and Wu520) and cytotoxicity(Reference Cheah, Ng and Ng521). It can also act as an antidepressant(Reference Nakamichi, Nakayama and Ishimoto522). The evidence for this comes from direct studies(Reference Yang, Lin and Wu520) and feeding experiments(Reference Nakamichi, Nakayama and Ishimoto522) in mice, as well as via the reduction of β-amyloid peptide in a transgenic C. elegans model(Reference Cheah, Ng and Ng521). As mentioned above, SLC22A4 polymorphisms are associated with ischaemic stroke(Reference Yamase, Horibe and Ueyama417).
Use of ergothioneine as an antioxidant in processed foodstuffs
Just as living beings exploit antioxidants, most foodstuffs can also be oxidised to produce taints, rancidity or other undesirable products(Reference Logan, Nienaber and Pan523–Reference Gülçin525), often via the Fenton reaction(Reference Kell208,Reference Perron and Brumaghim526) . ERG inhibits polyphenoloxidases(Reference Hanlon527), and thus ERG has been used in the feed of the shrimp Marsupenaeus japonicas to prevent melanosis during storage(Reference Encarnacion, Fagutao and Hirono528), while ERG-rich mushroom extract has also been used to prevent melanosis in post-harvest storage of the crab Chionoecetes japonicus (Reference Encarnacion, Fagutao and Hirayama529). Thus, one can also envisage a role for ERG, whether natural or added, in extending shelf lives and commercial value(Reference Nguyen, Nagasaka, Ohshima, Logan, Nienaber and Pan245,Reference Tang, Hoo and Tan328,Reference Pahila, Ishikawa and Ohshima475,Reference Encarnacion, Fagutao and Hirono528–Reference Duy Bao, Ohshima, Nienaber and Pan539) . The thermostability of ERG is of particular importance here.
Use of ergothioneine in cosmetics
Just as processed foodstuffs ‘age’, so do tissues such as the skin, and although the same principles apply(Reference Pérez-Sánchez, Barrajón-Catalán and Herranz-López540), it is common to refer to nutraceuticals that are also aimed at having cosmetic benefits as ‘cosmeceuticals’(Reference Lee541–Reference Taofiq, González-Paramás and Martins543). Here too, ERG has been widely used(Reference Taofiq, González-Paramás and Martins543–Reference Souyoul, Saussy and Lupo547), since much skin damage is caused by UV-mediated reactive oxygen species production(Reference Norins548); indeed, ERG is known as a skin protectant(Reference Dong, Damaghi and Kibitel240–Reference Markova, Karaman-Jurukovska and Dong244,Reference Hseu, Lo and Korivi549–Reference Bazela, Solyga-Zurek and Debowska551) .
Role of ergothioneine as a cofactor?
Although it is possible that the role of ERG lies simply in being an antioxidant capable of mopping up hydroxyl radicals and other reactive oxygen species, especially in prokaryotes(Reference Fahey36,Reference Trivedi, Singh and Bhat66,Reference Richard-Greenblatt, Bach and Adamson93,Reference Sao Emani, Williams and Wiid254,Reference Nakajima, Satoh and Yanashima255,Reference Ta, Buchmeier and Newton258–Reference Cumming, Chinta and Reddy260,Reference Sao Emani, Williams and Van Helden552,Reference Sao Emani, Williams and Van Helden553) , the roles of most other vitamins involve interaction with proteins, often as cofactors. This is also true for mycothiol(Reference Rawat and Av-Gay73,Reference Jothivasan and Hamilton554–Reference Newton, Buchmeier and Fahey556) , though that molecule can also serve as a signal and nutrient resource(Reference Bzymek, Newton and Ta557). However, despite many hypotheses(Reference Brummel558,Reference Brummel559) , the only example of ERG acting as a cofactor known to date is an involvement in the biosynthesis of lincomycin(Reference Wang, Zhao and Liu560,Reference Zhao, Wang and Xu561) . An early paper(Reference Goldberg562) implying an involvement of ERG in the maintenance of a reduced state of Fe in Hb, although apparently accurate, does not seem to have been followed up to date.
Conclusions
There is increasing awareness that health may be enhanced via the consumption of substances that either have no recommended daily intake or are taken at levels greater than normal, and such substances are commonly referred to as nutraceuticals. ERG, a potent and effective antioxidant, seems to be an important nutraceutical, and we rehearse a very broad literature, involving a great many cells, tissues and organisms, to that effect. The chief source of ERG in the human diet is mushrooms (usually the fruiting bodies of Basidiomycetes). The fact that a specific transporter known as SLC22A4 has evolved and been selected to effect ERG uptake in all known animals suggests strongly that ERG is of benefit to its consumers. While the evidence that ERG may be a useful nutraceutical as a preventive or palliative for various inflammatory diseases is extensive, it is mostly circumstantial rather than definitive, though many examples exist of the benefits of mushrooms in combating the results of oxidative stress.
Without mechanisms, finding that the concentration of a dietary metabolite X is low in disease Y does not mean that giving it might be of benefit in the prevention, delay or cure of that disease, although cases can clearly be made when X is a vitamin, or oxidative stress is known to be a damaging component of Y(Reference Kerley, McCarthy and Kell8,Reference Feng, Cheah and Ng348) . Thus far, we lack examples in which ERG is found both to be low in individuals with a particular syndrome and where exogenous administration effects functional improvements, although – as reviewed above – we often have one or the other.
To assess definitively any health benefits of ERG, the ‘gold standard’ of randomised controlled trials may take time and money, but – as with mushrooms(Reference Feeney, Miller and Roupas335,563) – are beginning. One trial with pure ERG has been registered(Reference Cheah564).
Note added in proof
A recent paper indicates that ERG relieves the effects seen in a rat model of the pregnancy disorder pre-eclampsia(Reference Williamson, McCarthy and Manna597).
Acknowledgements
We thank the Biotechnology and Biological Sciences Research Council (BBSRC) (grants no. BB/R000093/1 and no. BB/P009042/1) and the Novo Nordisk Foundation (grant no. NNF10CC1016517) for financial support.
D. B. K. decided to bring together the multifaceted contributions of the various authors listed. Their previous contributions to ergothioneine and antioxidant biology may be seen in the references cited. All authors contributed to and approved the final manuscript.
I. B., S. A. v. d. H. and D. B. K. are named inventors on a patent application involving the biotechnological production of l-(+)-ergothioneine in yeast. For the other authors, there are no conflicts of interest.