Published online by Cambridge University Press: 19 March 2013
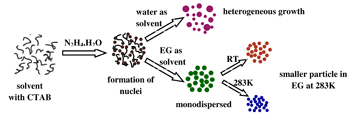
We have prepared stable ultrafine narrow dispersed copper nanoparticles (Cu-NPs) using a facile chemical reduction technique below room temperature (300 K), without any template. X-ray diffraction and high-resolution transmission electron microscopy studies reveal the growth of highly crystalline Cu-NPs with an average diameter of 2.2 nm. Interestingly, these Cu-NPs demonstrate both interband electronic transitions along with usual surface plasmon resonance, a unique phenomenon previously unobserved in any noble metal nanoparticles. These Cu-NPs do not get oxidized easily and could be suitable candidates for different optical devices, heat transfer liquids, and biological applications.