Article contents
The state of platinum in pyrrhotite: X-ray absorption spectroscopy study and implications for the role of Fe sulfides as platinum carriers
Published online by Cambridge University Press: 26 October 2021
Abstract
Pyrrhotite Fe1–xS is the main sulfide component of platinum-group element (PGE) ores and commonly contains from a few tenths to a few dozen ppm of disseminated Pt. Here we report an X-ray absorption spectroscopy investigation into the state of Pt in synthetic pyrrhotite in combination with theoretical spectra modelling. The pyrrhotite crystals were obtained by means of the salt flux technique, using a eutectic mixture of alkali metal halides as the transport media. Analysis of the chemical composition of synthesised crystals showed that an increase of the temperature and sulfur fugacity yields higher concentrations of Pt in pyrrhotite. The Pt content reached 0.6 wt.% at the maximum temperature and sulfur fugacity (t = 720°C, log $f_{{\rm S}_ 2}$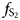 = –0.1) achieved in a Pt-saturated system. X-ray absorption near-edge structure (XANES) analysis of Pt L3-edge spectra revealed that Pt is present in pyrrhotite in the 4+ and 2+ ‘formal’ oxidation states. Theoretical modelling of XANES and interpretation of extended X-ray absorption fine structure (EXAFS) spectra showed that Pt4+ substitutes for Fe in the crystal lattice of pyrrhotite, whereas Pt2+ forms PtS-like clusters disseminated in the pyrrhotite matrix. Atoms of isomorphous Pt4+ are surrounded by 6 S atoms at a distance of 2.39 ± 0.02 Å. According to theoretical simulations using the FDMNES program, the second coordination sphere of the solid-solution Pt contains one vacancy in the Fe sublattice within the Fe-layer. The Pt2+S-like clusters can be considered as a quench product. High sulfur fugacity stabilises the solid-solution Pt and prevents the formation of the PtS-like clusters during cooling. The maximum content of the solid-solution Pt in pyrrhotite is ca. 50 times lower than in pyrite and can be approximated by a straight line in the log C(Pt) vs. 1/T plot, it increases from 1 ppm at 350°C to 3 wt.% at 900°C.
= –0.1) achieved in a Pt-saturated system. X-ray absorption near-edge structure (XANES) analysis of Pt L3-edge spectra revealed that Pt is present in pyrrhotite in the 4+ and 2+ ‘formal’ oxidation states. Theoretical modelling of XANES and interpretation of extended X-ray absorption fine structure (EXAFS) spectra showed that Pt4+ substitutes for Fe in the crystal lattice of pyrrhotite, whereas Pt2+ forms PtS-like clusters disseminated in the pyrrhotite matrix. Atoms of isomorphous Pt4+ are surrounded by 6 S atoms at a distance of 2.39 ± 0.02 Å. According to theoretical simulations using the FDMNES program, the second coordination sphere of the solid-solution Pt contains one vacancy in the Fe sublattice within the Fe-layer. The Pt2+S-like clusters can be considered as a quench product. High sulfur fugacity stabilises the solid-solution Pt and prevents the formation of the PtS-like clusters during cooling. The maximum content of the solid-solution Pt in pyrrhotite is ca. 50 times lower than in pyrite and can be approximated by a straight line in the log C(Pt) vs. 1/T plot, it increases from 1 ppm at 350°C to 3 wt.% at 900°C.
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press on behalf of The Mineralogical Society of Great Britain and Ireland
Footnotes
Associate Editor: František Laufek
References
- 7
- Cited by


