Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Chukanov, Nikita
Shendrik, Roman
Vigasina, Marina
Pekov, Igor
Sapozhnikov, Anatoly
Shcherbakov, Vasily
and
Varlamov, Dmitry
2022.
Crystal Chemistry, Isomorphism, and Thermal Conversions of Extra-Framework Components in Sodalite-Group Minerals.
Minerals,
Vol. 12,
Issue. 7,
p.
887.
Chukanov, Nikita V.
Vigasina, Marina F.
Shendrik, Roman Yu.
Varlamov, Dmitry A.
Pekov, Igor V.
and
Zubkova, Natalia V.
2022.
Nature and Isomorphism of Extra-Framework Components in Cancrinite- and Sodalite-Related Minerals: New Data.
Minerals,
Vol. 12,
Issue. 6,
p.
729.
Chukanov, Nikita
Shchipalkina, Nadezhda
Shendrik, Roman
Vigasina, Marina
Tauson, Vladimir
Lipko, Sergey
Varlamov, Dmitry
Shcherbakov, Vasiliy
Sapozhnikov, Anatoly
Kasatkin, Anatoly
Zubkova, Natalia
and
Pekov, Igor
2022.
Isomorphism and Mutual Transformations of S-Bearing Components in Feldspathoids with Microporous Structures.
Minerals,
Vol. 12,
Issue. 11,
p.
1456.
Расцветаева, Р.К.
2022.
И содалиты тоже разные: открытие двух новых минералов группы содалита, "Природа".
Priroda,
p.
37.
Gritsenko, Yu. D.
Eremina, E. N.
Vigasina, M. F.
Vyatkin, S. V.
Ogorodova, L. P.
Maltsev, V. V.
and
Melchakova, L. V.
2023.
Sodalite: Spectroscopic and Thermochemical Investigations.
Geochemistry International,
Vol. 61,
Issue. 7,
p.
735.
Chukanov, Nikita V.
Sapozhnikov, Anatoly N.
Kaneva, Ekaterina V.
Varlamov, Dmitry A.
and
Vigasina, Marina F.
2023.
Bystrite, Na7Ca(Al6Si6O24)S52–Cl–: formula redefinition and relationships with other four-layer cancrinite-group minerals.
Mineralogical Magazine,
Vol. 87,
Issue. 3,
p.
455.
Pekov, Igor V.
and
Pushcharovsky, Dmitry Yu.
2023.
Celebrating the International Year of Mineralogy.
p.
69.
Fedyaeva, Maria
Lepeshkin, Sergey
and
Oganov, Artem R.
2023.
Stability of sulfur molecules and insights into sulfur allotropy.
Physical Chemistry Chemical Physics,
Vol. 25,
Issue. 13,
p.
9294.
Vigasina, M. F.
Vyatkin, S. V,
Ogorodova, L. P.
Maltsev, V. V.
Melchakova, L. V.
Gritsenko, Yu. D.
and
Eremina, E. N.
2023.
SODALITE: SPECTROSCOPIC AND THERMOCHEMICAL INVESTIGATIONS.
Геохимия,
Vol. 68,
Issue. 7,
p.
720.
Bolotina, Nadezhda B.
Sapozhnikov, Anatoly N.
Chukanov, Nikita V.
and
Vigasina, Marina F.
2023.
Structure Modulations and Symmetry of Lazurite-Related Sodalite-Group Minerals.
Crystals,
Vol. 13,
Issue. 5,
p.
768.
Sapozhnikov, Anatoly N.
Bolotina, Nadezhda B.
Chukanov, Nikita V.
Shendrik, Roman Yu.
Kaneva, Ekaterina V.
Vigasina, Marina F.
Ivanova, Larisa A.
Tauson, Vladimir L.
and
Lipko, Sergey V.
2023.
Slyudyankaite, Na28Ca4(Si24Al24O96)(SO4)6(S6)1/3(CO2)·2H2O, a new sodalite-group mineral from the Malo-Bystrinskoe lazurite deposit, Baikal Lake area, Russia.
American Mineralogist,
Vol. 108,
Issue. 9,
p.
1805.
Zubkova, N. V.
Chukanov, N. V.
Varlamov, D. A.
Vigasina, M. F.
Pekov, I. V.
Ksenofontov, D. A.
and
Pushcharovsky, D. Yu.
2023.
A Sulfite-Bearing Analog of Marinellite.
Geology of Ore Deposits,
Vol. 65,
Issue. 7,
p.
782.
Расцветаева, Р.К.
2023.
А почему он такой зеленый? Открытие слюдянкаита — нового минерала группы содалита, "Природа".
Priroda,
p.
38.
Chukanov, Nikita V.
Sapozhnikov, Anatoly N.
Shendrik, Roman Yu.
Zubkova, Natalia V.
Vigasina, Marina F.
Potekhina, Nadezhda V.
Ksenofontov, Dmitry A.
and
Pekov, Igor V.
2023.
Crystal Chemistry, Thermal and Radiation-Induced Conversions and Indicatory Significance of S-Bearing Groups in Balliranoite.
Minerals,
Vol. 13,
Issue. 6,
p.
822.
Chukanov, Nikita V.
Aksenov, Sergey M.
and
Pekov, Igor V.
2023.
Infrared spectroscopy as a tool for the analysis of framework topology and extra-framework components in microporous cancrinite- and sodalite-related aluminosilicates.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,
Vol. 287,
Issue. ,
p.
121993.
Shchipalkina, Nadezhda V.
Vereshchagin, Oleg S.
Chukanov, Nikita V.
Gorelova, Liudmila A.
Bocharov, Vladimir N.
and
Pekov, Igor V.
2023.
High-temperature behavior of the UV-luminescent sodalite-type natural compound Na8(Al6Si6O24)(HS)2: Comparative study by in situ single-crystal X-ray diffraction and Raman spectroscopy.
Journal of Solid State Chemistry,
Vol. 323,
Issue. ,
p.
124067.
Pekov, I. V.
Chukanov, N. V.
Shcherbakov, V. D.
Vigasina, М. F.
Shendrik, R. Yu.
Sandalov, F. D.
Vyatkin, S. V.
and
Turchkova, А. G.
2024.
Rock-forming feldspathoids of the sodalite–sapozhnikovite series from the Lovozero alkaline complex (Kola peninsula, Russia): isomorphism, thermal and radiation-induced transformations and genetic mineralogy.
Zapiski Vserossijskogo mineralogičeskogo obŝestva,
Vol. CLIII,
Issue. 1,
p.
12.
Chukanov, Nikita V.
and
Aksenov, Sergey M.
2024.
Structural Features, Chemical Diversity, and Physical Properties of Microporous Sodalite-Type Materials: A Review.
International Journal of Molecular Sciences,
Vol. 25,
Issue. 18,
p.
10218.
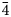 3n, with a = 8.9146(1) Å, V = 708.45(2) Å3 and Z = 1. The new mineral is isostructural with sodalite. The strongest lines of the powder X-ray diffraction pattern [d, Å (I, %) (hkl)] are: 6.30 (37) (110), 3.638 (100) (211), 2.821 (14) (310), 2.572 (18) (222), 2.382 (16) (321) and 2.101 (29) (411). The mineral is named in honour of the Russian mineralogist and crystallographer Dr. Anatoly Nikolaevich Sapozhnikov (b. 1946).
3n, with a = 8.9146(1) Å, V = 708.45(2) Å3 and Z = 1. The new mineral is isostructural with sodalite. The strongest lines of the powder X-ray diffraction pattern [d, Å (I, %) (hkl)] are: 6.30 (37) (110), 3.638 (100) (211), 2.821 (14) (310), 2.572 (18) (222), 2.382 (16) (321) and 2.101 (29) (411). The mineral is named in honour of the Russian mineralogist and crystallographer Dr. Anatoly Nikolaevich Sapozhnikov (b. 1946).

