Article contents
Lussierite, a new sodium uranyl sulfate mineral with bidentate UO7–SO4 linkage from the Blue Lizard mine, San Juan County, Utah, USA
Published online by Cambridge University Press: 03 June 2019
Abstract
The new mineral lussierite (IMA2018-101), Na10[(UO2)(SO4)4](SO4)2(H2O)3, was found in the Blue Lizard mine, San Juan County, Utah, USA, where it occurs as pale green–yellow prisms or blades in a secondary assemblage with belakovskiite, ferrinatrite, halite, ivsite, metavoltine and thénardite. The streak is white and the fluorescence is bright cyan under 365 nm ultraviolet light. Crystals are transparent with vitreous lustre. The tenacity is brittle, the Mohs hardness is 2½, the fracture is irregular and no cleavage was observed. The mineral is easily soluble in H2O and has a measured density of 2.87(2) g cm–3. Lussierite is optically biaxial (+), with α = 1.493(1), β = 1.505(1) and γ = 1.518(1) (white light); 2Vmeas. = 88(1)°; dispersion is r > v, moderate; pleochroism: X = colourless, Y and Z = green yellow (X < Y ≈ Z); optical orientation: X = b, Z ∧ a = 44° in obtuse β. Electron microprobe analyses (wavelength-dispersive spectroscopy mode) provided Na10(U0.99O2)(S1.00O4)6·3H2O (+0.06 H for charge balance). The five strongest X-ray powder diffraction lines are [dobs Å(I)(hkl)]: 6.69(95)(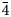 $\bar{1}$11,130), 4.814(100)(150,002,060), 3.461(83)(171,
$\bar{1}$11,130), 4.814(100)(150,002,060), 3.461(83)(171,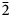 $\bar{2}$02), 2.955(81)(113,330) and 2.882(74)(
$\bar{2}$02), 2.955(81)(113,330) and 2.882(74)(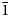 $\bar{1}$91,311,191,0·10·0). Lussierite is monoclinic, Cc, a = 9.3134(4), b = 28.7501(11), c = 9.6346(7) Å, β = 93.442(7)°, V = 2575.1(2) Å3 and Z = 4. The structure (R1 = 0.0298 for 5202 I > 2σI) contains a [(UO2)(SO4)4]6– uranyl sulfate cluster in which one SO4 tetrahedron shares an edge (bidentate linkage) with the UO7 pentagonal bipyramid. The uranyl sulfate clusters occur in layers and are linked through a complex network of bonds involving Na+ cations, isolated SO4 tetrahedra and isolated H2O groups.
$\bar{1}$91,311,191,0·10·0). Lussierite is monoclinic, Cc, a = 9.3134(4), b = 28.7501(11), c = 9.6346(7) Å, β = 93.442(7)°, V = 2575.1(2) Å3 and Z = 4. The structure (R1 = 0.0298 for 5202 I > 2σI) contains a [(UO2)(SO4)4]6– uranyl sulfate cluster in which one SO4 tetrahedron shares an edge (bidentate linkage) with the UO7 pentagonal bipyramid. The uranyl sulfate clusters occur in layers and are linked through a complex network of bonds involving Na+ cations, isolated SO4 tetrahedra and isolated H2O groups.
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2019
Footnotes
Current address: Section of Minerals, Carnegie Museum of Natural History, 4400 Forbes Avenue, Pittsburgh, PA 15213, USA
Associate Editor: Daniel Atencio
References
- 6
- Cited by


