Published online by Cambridge University Press: 05 July 2018
Klajite, MnCu4(AsO4)2(AsO3OH)2(H2O)10, the Mn-Cu-bearing member of the lindackerite group, was found in Jáchymov, Czech Republic, as the second world occurrence. It is associated with ondrušite and other arsenate minerals growing on the quartz gangue with disseminated primary sulfides, namely tennantite and chalcopyrite. Electron-microprobe data showed klajite aggregates to be chemically inhomogeneous at larger scales, varying from Mn-Ca-rich to Cu-rich domains. The chemical composition of the the Mn-rich parts of aggregates can be expressed by the empirical formula (Mn0.46Ca0.22Cu0.07Mg0.02)∑0.77(Cu3.82Mg0.14Ca0.03Zn0.01)∑4.00(As1.94Si0.06)∑2.00O8[AsO2.73(OH)1.27]2(H2O)10 (mean of seven representative spots; calculated on the basis of As + Si + P = 4 a.p.f.u. (atoms per formula unit) and 10 H2O from ideal stoichiometry), showing a slight cationic deficiency at the key Me-site. According to single-crystal X-ray diffraction, klajite from Jáchymov is triclinic, P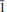 , with a = 6.4298(8), b = 7.9716(8), c = 10.707(2) Å, α = 85.737(12)°, β = 80.994(13)°, γ = 84.982(10)°, and V = 538.85(14) Å3, Z = 1. The crystal structure was refined to R1 = 0.0628 for 1034 unique observed reflections (with Iobs > 3σ(I)), confirming that klajite (Mn-Cu member) and ondrušite (Ca-Cu member) are isostructural. The current data-set allowed determination of the positions of several hydrogen atoms. Discussion on hydrogen bonding networks in the structure of klajite as well as detailed bond-valence analysis are provided.
, with a = 6.4298(8), b = 7.9716(8), c = 10.707(2) Å, α = 85.737(12)°, β = 80.994(13)°, γ = 84.982(10)°, and V = 538.85(14) Å3, Z = 1. The crystal structure was refined to R1 = 0.0628 for 1034 unique observed reflections (with Iobs > 3σ(I)), confirming that klajite (Mn-Cu member) and ondrušite (Ca-Cu member) are isostructural. The current data-set allowed determination of the positions of several hydrogen atoms. Discussion on hydrogen bonding networks in the structure of klajite as well as detailed bond-valence analysis are provided.