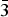Introduction
‘Jixianite’ used to be an approved mineral found in the Yanhe tungsten deposit in Jixian County, Hebei (currently Jizhou District in Tianjin City), China, by Liu (Reference Liu1979). It was an oxide containing W and Pb components and was approved by the International Mineralogical Association Commission on New Minerals and Mineral Names (IMA−CNMMN) at that time. However, because of the mineral's tiny crystal size, the single crystal structure data could not be obtained. According to the powder-diffraction data, the diffraction characteristics are similar to those of pyrochlore-supergroup minerals, so it was classified as a pyrochlore-group mineral containing W and Pb, with the formula Pb2(W,Fe)2(O,OH)7.
The general formula for the pyrochlore supergroup is A 2−mB 2X 6−wY 1−n (Atencio et al., Reference Atencio, Andrade, Christy, Giere and Kartashov2010). In 2010, based on the proposed new scheme for the nomenclature of the pyrochlore supergroup, which was approved by the IMA−CNMNC, ‘jixianite’ was discredited due to the absence of a detailed crystal structure study and lack of information about the Y site dominant anion (Atencio et al., Reference Atencio, Andrade, Christy, Giere and Kartashov2010). More recently, the status of the mineral's name has been changed from ‘discredited’ to ‘questionable’ pending further research (Christy and Atencio, Reference Christy and Atencio2013).
Recently, we obtained a cotype specimen from Mr. Liu Jianchang who first found ‘jixianite’ in 1979 and we also collected new samples from the type locality. We selected 156 fragments from the cotype specimen to make an epoxy block for single-side polishing, including the residual part of the fragment used for structural data collection. Infrared spectroscopy, Raman spectroscopy and electron microprobe analysis were all performed on the polished surface of this sample group. Through this investigation, we discovered two members of the elsmoreite group within the pyrochlore supergroup in the cotype specimen of ‘jixianite’, including hydroplumboelsmoreite (IMA21-C, Miyawaki, Reference Miyawaki, Hatert, Pasero and Mills2021), which replaces ‘jixianite’, and a potential new mineral provisionally named ‘hydroelsmoreite’ (IMA2019-066, pending resubmission).
In this study, a 1.2 kW high-power microfocus rotating anode X-ray source and a hybrid pixel array detector single-crystal diffractometer were used to analyse the structure, and the single-crystal diffraction data were obtained for a small crystal (10 μm × 10 μm × 10 μm in size). The results show that hydroplumboelsmoreite crystallises in the cubic system, space group Fd $\bar{3}$![]() m, with lattice parameters of a = 10.3377(5) Å, V = 1104.77(16) Å3 and Z = 8, so it has a pyrochlore type structure.
m, with lattice parameters of a = 10.3377(5) Å, V = 1104.77(16) Å3 and Z = 8, so it has a pyrochlore type structure.
The new electron-microprobe analyses carried out on hydroplumboelsmoreite are more accurate than the previous analyses, and were used to determine a simplified formula: (Pb,□)2(W, Fe3+)2O6(H2O). According to the nomenclature of the pyrochlore supergroup, the mineral ‘jixianite’ is redefined as hydroplumboelsmoreite as it corresponds to a member of the elsmoreite group with Pb dominant in the A site and H2O dominant in the Y site.
Hydroplumboelsmoreite and its name have been approved by the IMA−CNMNC and replaces ‘jixianite’. The type specimen of hydroplumboelsmoreite has been registered within the collections of the Geological Museum of China (Xisi Yangrou hutong No. 15, Xicheng District, Beijing), China, under catalogue number M16125.
Occurrence and paragenesis
The mineral was discovered in the oxidised section of hydrothermal tungsten-bearing lead deposits in the Yanhe tungsten deposit in Jixian County, Hebei Province, China, which is now called the Jizhou District in Tianjin City (40°1’54.8’’N, 117°17’22.4’’E)
The Jixian Yanhe tungsten deposit is situated in the middle of the Yanshan subsidence belt, the west wing of the Malanyu anticline, the southwest section of the Panshan short-axis anticline, and the southern border of the contact zone of the Panshan rock body (porphyritic quartz monzonite) (Fig. 1). The deposit is a high-temperature hydrothermal tungsten-bearing quartz vein type deposit (Wang et al., Reference Wang, Duo, Tu, Chen and Wen2013). The tungsten mineralisation was related closely to magmatic activity and occurs in the inner contact zone of the rock. The metallogenic rock is mainly Late Indosinian intermediate-felsic rock, and the ore-bearing surrounding rocks are quartz monzonite and medium-to-fine-grained granite (Wen et al., Reference Wen, Zhu, Zhang, Duo and Wang2015). The occurrence, morphology, and scale of the tungsten-bearing quartz veins are strictly controlled by the lithology and structural fractures (Wen et al., Reference Wen, Zhu, Zhang, Duo and Wang2015). More than 290 quartz veins have been found in this area (Wang et al., Reference Wang, Duo, Tu, Chen and Wen2013).
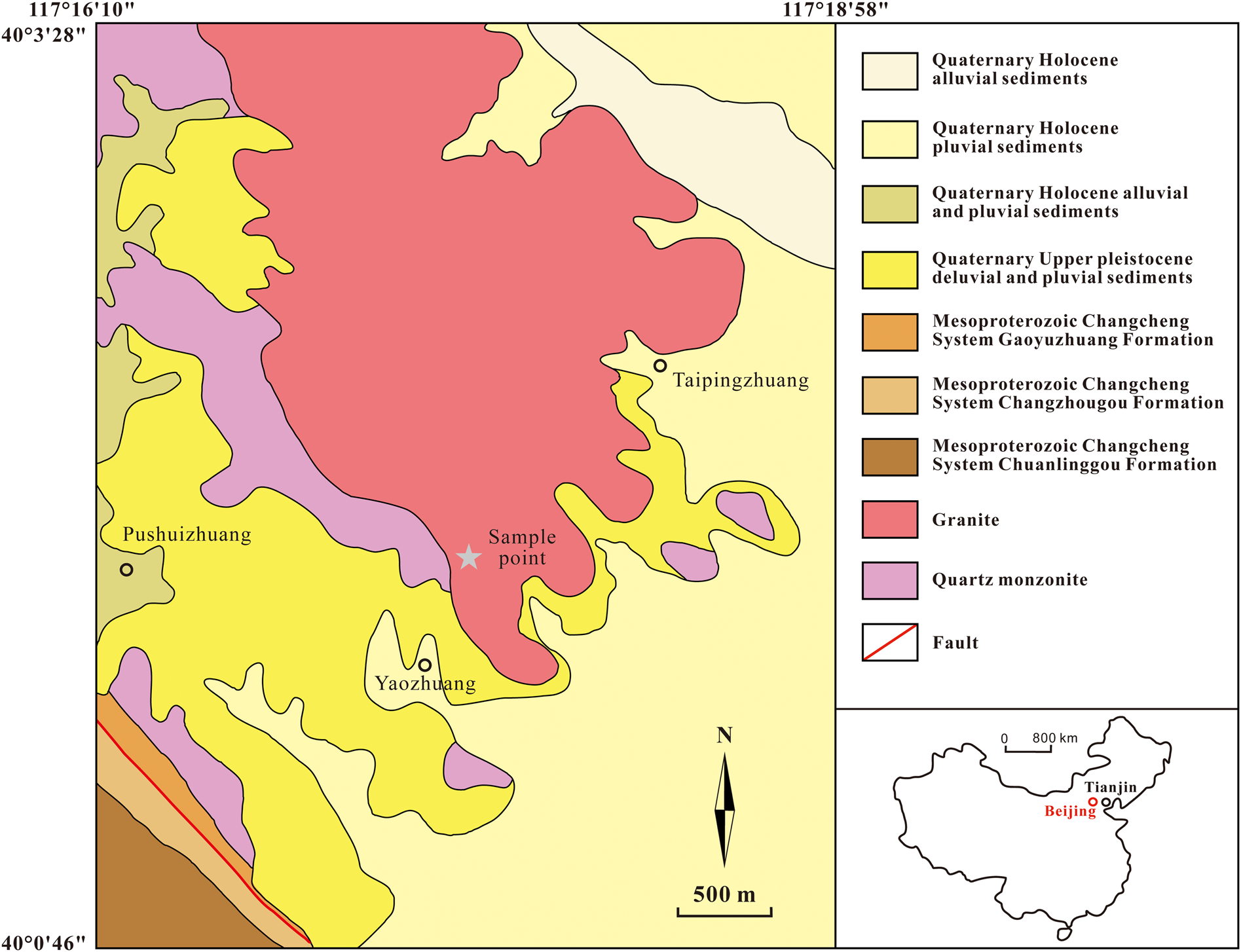
Fig. 1. Geological map of the locality.
Hydroplumboelsmoreite occurs in the oxidised section of the tungsten-bearing quartz veins (Liu, Reference Liu1979). The primary minerals in the oxidised zone include quartz, pyrite, ferberite, cassiterite, chalcopyrite, scheelite, acanthite, silver and copper. The secondary minerals found nearby mainly include muscovite, bismutite, raspite, wulfenite, malachite, covellite, hydroplumboelsmoreite, and ‘hydroelsmoreite’ (under investigation).
Hydroplumboelsmoreite is an uncommon secondary mineral formed in the oxidised zone of the hydrothermal tungsten-bearing lead deposits. It mostly occurs as microcrystalline to cryptocrystalline aggregates, with an earthy, honeycombed, or crusty appearance (Fig. 2a), and the single crystals are tiny (Fig. 2b), exhibiting the characteristics of a hypergene mineral. The mineral is commonly intergrown with raspite (Fig. 2c,d) and ‘hydroelsmoreite’, and may be formed as a secondary mineral after scheelite, ferberite and wulfenite.

Fig. 2. Aggregate grains of hydroplumboelsmoreite: (a–c) aggregate grains of hydroplumboelsmoreite (Hpe) and (d) paragenetic raspite (Rsp) (symbols according to Warr, Reference Warr2021).
Appearance and physical and optical properties
Generally, the aggregations reach 300 μm in size, but crystals are <20 μm across (Fig. 2a,b). Monocrystals are rare, and they are subhedral or allotriomorphic granular, with the dominant form {111}. The aggregates are yellow or reddish brown (Fig. 2a). The crystals are colourless with a white streak, translucent with a greasy lustre, brittle with a conchoidal fracture, and no apparent twinning, cleavage, or parting was observed.
Hydroplumboelsmoreite is isotropic, and the refractive index calculated based on its chemical composition and Gladstone–Dale relationship (N = K d + 1, K from Mandarino, Reference Mandarino1981) is 2.29. No fluorescence was observed.
The density could not be measured because the single-crystal particles of this mineral are tiny and are generally intergrown with other minerals. However, the density calculated based on the empirical formula and the unit cell parameters refined from the single-crystal X-ray diffraction data is 7.47 g⋅cm−3. Vickers microhardness tests generally gave values of 278.59–302.3 kg⋅mm−2 (HV0.1kgf), and the Mohs hardness estimated by the VHN test is ~4½–5.
Spectroscopy
Infrared spectrum
The infrared (IR) spectrum of a polished surface of hydroplumboelsmoreite was obtained using a Bruker27 IR microscope equipped with a liquid nitrogen-cooled detector in reflection mode. The spectrum in the range of 4000–400 cm–1 was collected by averaging 32 s scans at a resolution of 4 cm–1. The IR spectrum is shown in Fig. 3. The samples were stored at 110°C for 2 hours before the analysis to avoid the effect of adsorbed water on the test results. The 3552 cm−1(strong) and 1631 cm−1(medium) bands represent the stretching vibration and bending vibration of H2O, respectively. The IR absorption related to the W=O stretching vibration is represented by two peaks at 916(weak) and 859(weak) (Gadsden, Reference Gadsden1975; Farmer, Reference Farmer1982). All of the other bands in the 400–700 cm−1 range are due to the vibrations of the elsmoreite-type framework.
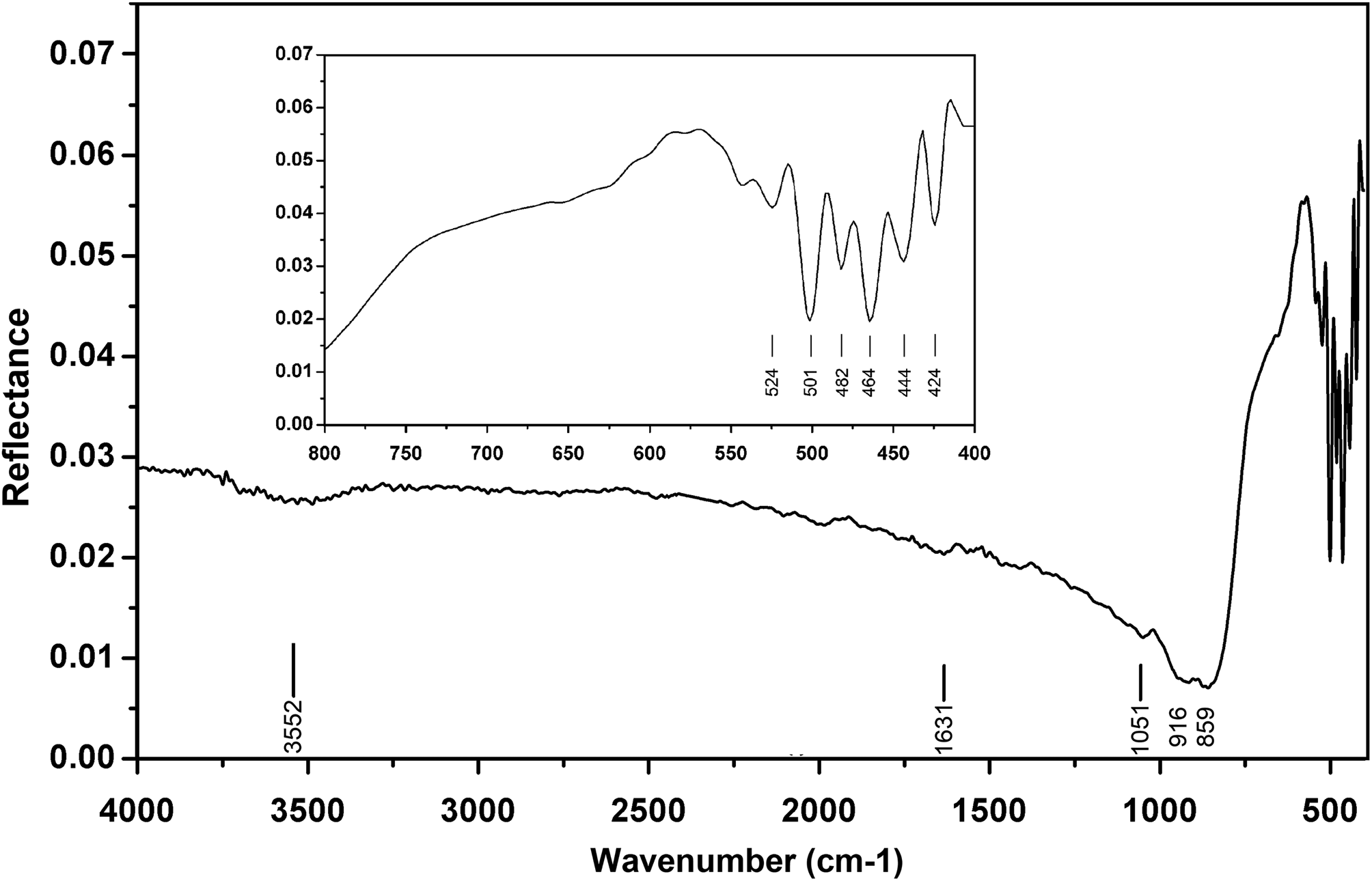
Fig. 3. Infrared spectrum of hydroplumboelsmoreite.
Raman spectrum
The Raman spectrum was obtained using a RENISHAW in Via-Reflex microscope Raman spectrometer equipped with a 532 nm laser, in reflection mode, and the data acquisition time was 10 s. The Raman spectrum is shown in Fig. 4. The Raman spectrum of hydroplumboelsmoreite is characterised by an intense band at 891 cm−1 (W=O stretching) (Mills et al., Reference Mills, Christy, Kampf, Birch and Kasatkin2017). The bands at 695 and 501 cm−1 correspond to the O–W–O stretching modes, while the bands at 436 and 362 cm−1 correspond to the O–W–O bending modes (Gu et al., Reference Gu, Ma, Zhai, Gao, Yang and Yao2006). The bands in the 100–350 cm−1 region are assigned to the lattice modes.
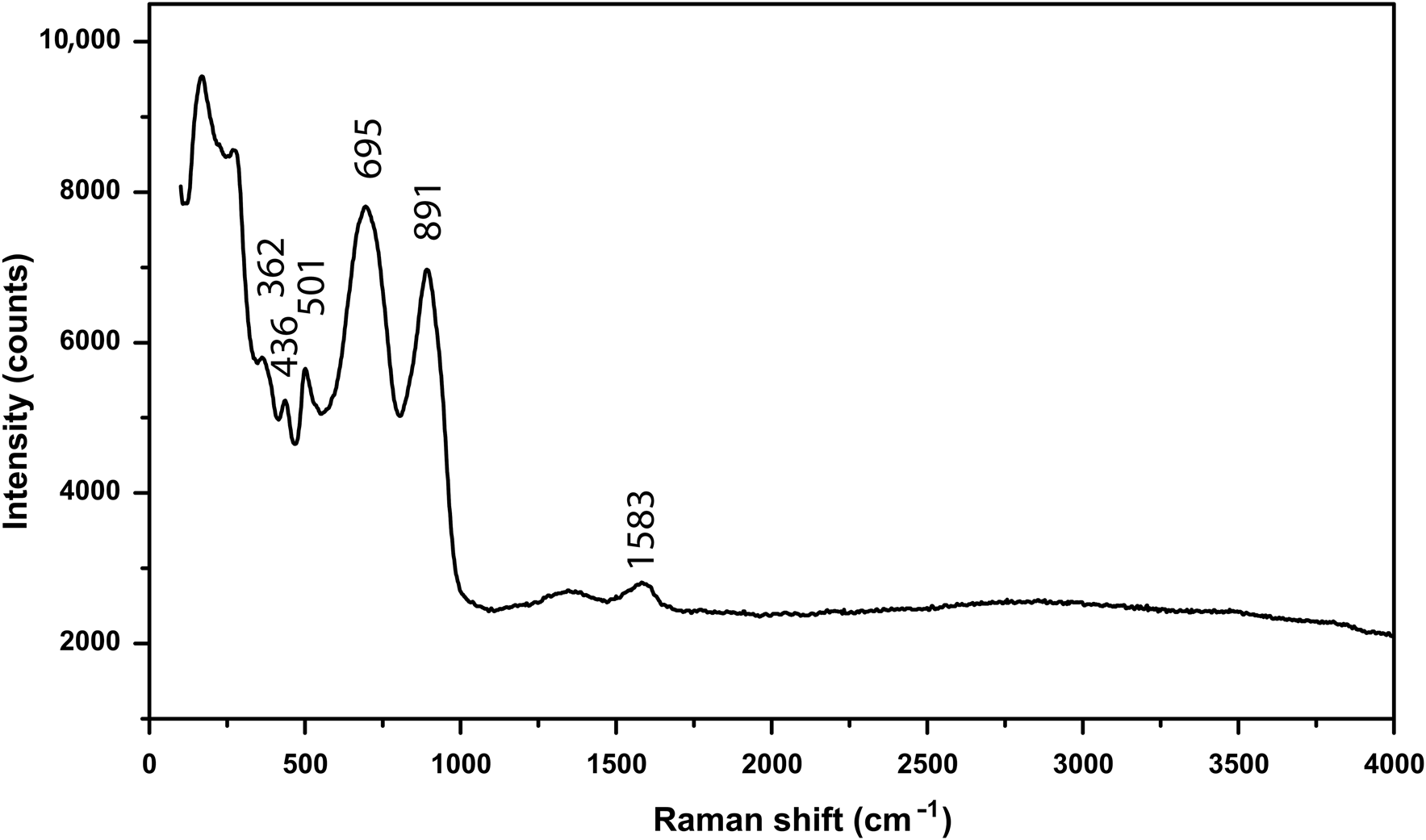
Fig. 4. Raman spectrum of hydroplumboelsmoreite.
The band at 1583 cm−1 is an indicator of molecular H2O, which is consistent with the IR spectrum. There is no obvious O–H stretching vibration band between 3400 and 3500 cm−1.
Chemical composition
An average of ten spot-analyses was obtained using an electron microprobe (JEOL JXA–8100, wavelength-dispersive spectrometry mode (WDS), 20 kV, 10 nA and 2 μm beam diameter). The analytical data for hydroplumboelsmoreite and ‘hydroelsmoreite’, and the reported data for ‘jixianite’ are summarised in Table 1. It was found that the contents and total amounts of the different components of hydroplumboelsmoreite and ‘hydroelsmoreite’ are significantly different while the hydroplumboelsmoreite data are rather similar to those for ‘jixianite’.
Table 1. Chemical data (in wt.%) for hydroplumboelsmoreite, ‘hydroelsmoreite’ and ‘jixianite’.
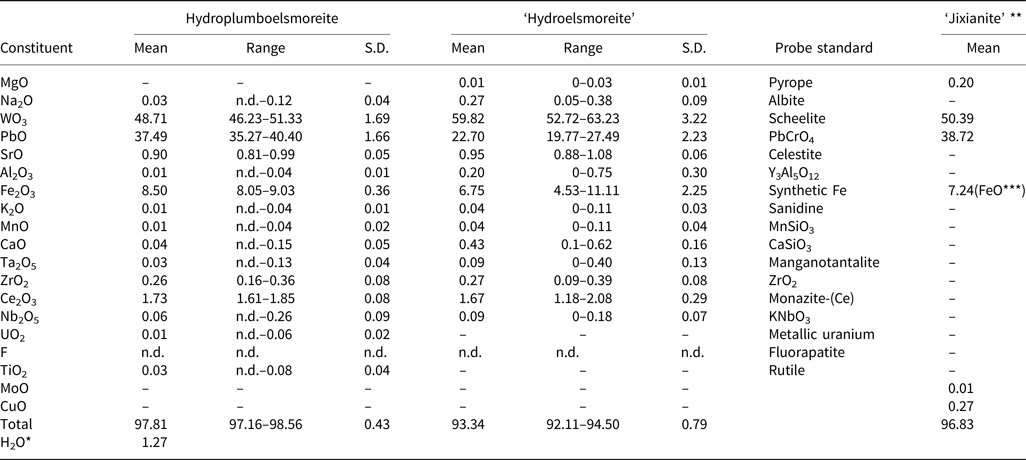
Notes: ‘hydroelsmoreite’ and ‘jixianite’ are not valid minerals approved by the IMA (Pasero, Reference Pasero2021). S.D. = standard deviation; n.d. = not detected; ‘–’ = not analysed
* H2O calculated by structure refinement; **Data from Liu (Reference Liu1979); ***FeO = Fe2O3 + FeO
The samples were stored again, at 110°C for 2 hours before the compositional analysis to avoid the effect of adsorbed water on the test results. Due to the very small particle-size of the hydroplumboelsmoreite, direct determination of H2O was not possible. The H2O was calculated based on the structure refinement. The presence of molecular H2O and the absence of OH groups were confirmed by the infrared (IR) and Raman spectra. The dominant occupant of the Y site (O2) was H2O, which is consistent with the structure refinement, the bond valence sum, and the chemical composition analysis.
Given the known occurrence of hydroplumboelsmoreite in the oxidised zone, the appearance of typical supergene characteristics, the low scattering factor for the W1 site based on the structure refinement, and based on previous studies of elsmoreite-group minerals, all of the Fe was assumed to be trivalent and was assigned to the B site. Ce, Sr, Na and Ca were assumed to be confined to the A site.
On the basis of the results from the electron microscope analyses and crystal structure refinement (see below), the empirical formula is (Pb1.05Sr0.05Ce3+0.07Na0.01□0.82)Σ2.00(W1.32Fe3+0.67Zr0.01)Σ2.00O6[(H2O)0.43O0.19□0.38]Σ1.00, calculated on the basis of B = 2.
Moreover, because hydroplumboelsmoreite and ‘hydroelsmoreite’ are indistinguishable in terms of appearance, it is not possible to determine whether the chemical data for ‘jixianite’ (Liu, Reference Liu1979) derived via wet-chemical analysis using larger amounts of subsample are accurate or not, and we speculate that the sample analysed may have been a mixture of raspite, hydroplumboelsmoreite and ‘hydroelsmoreite’.
X-ray diffraction and crystallography
Powder X-ray diffraction
The powder X-ray diffraction data (Table 2) were obtained using a Rigaku Oxford Diffraction XtaLAB PRO-007HF (MoKα, 50 kV and 24 mA) by the powder rotations method, from the 0.2 mm × 0.2 mm × 0.2 mm aggregated fragment used for all the test. The powder X-ray diffraction pattern of hydroplumboelsmoreite (MoKα) is shown in Fig. 5. The five strongest powder X-ray diffraction lines [d in Å(I)(hkl)] are 6.070(28)(111), 3.012(100)(222), 2.603(32)(004), 1.836(35)(044) and 1.568(30)(226). The unit cell parameters refined from the powder-diffraction data using the Chekcell software (Jean and Bernard, Reference Jean and Bernard2001) are as follows: cubic, Fd $\bar{3}$![]() m (#227), a = 10.388(11) Å, V = 1121(3) Å3 and Z = 8.
m (#227), a = 10.388(11) Å, V = 1121(3) Å3 and Z = 8.
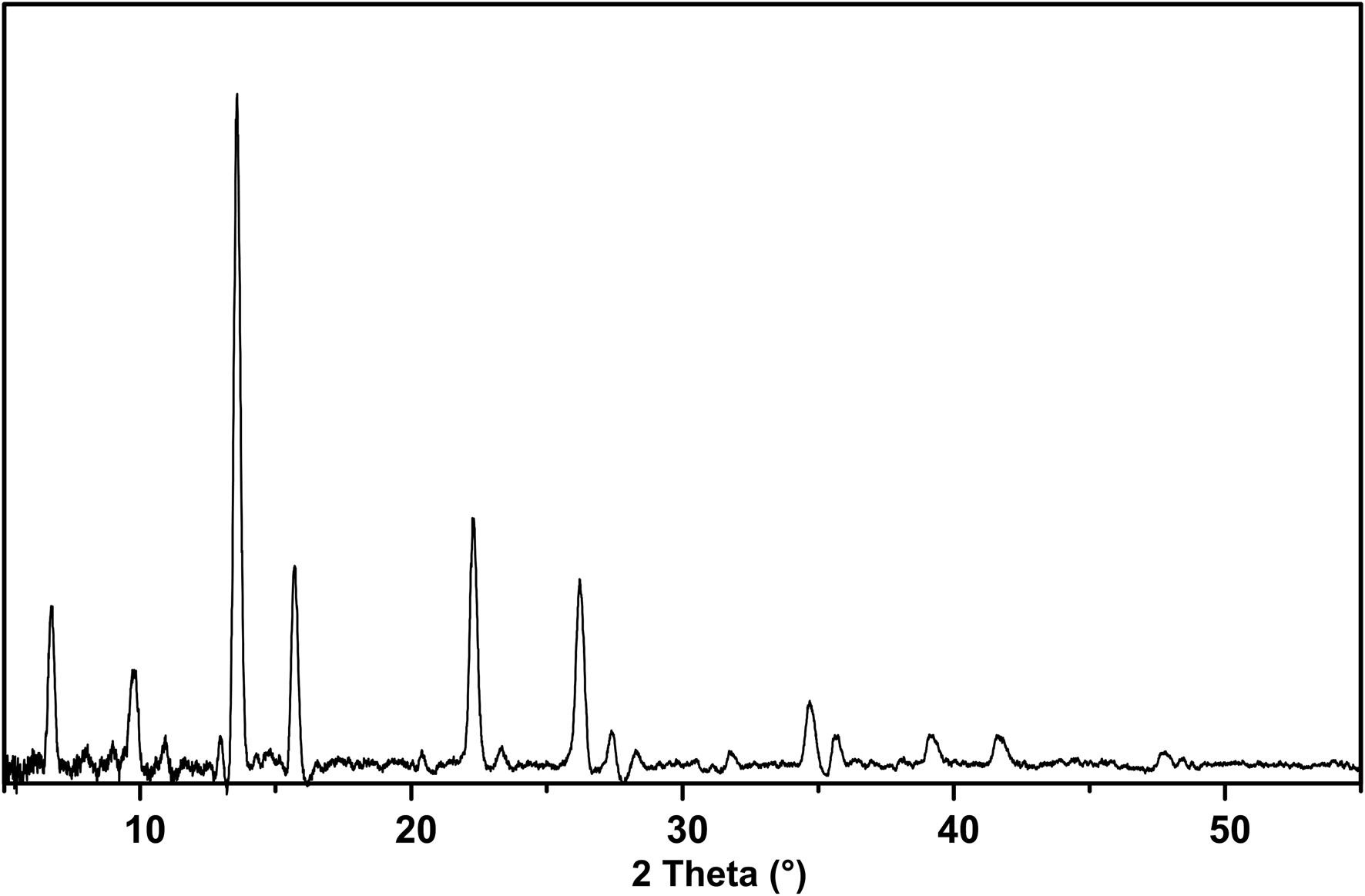
Fig. 5. Powder X-ray diffraction pattern of hydroplumboelsmoreite (MoKα).
Table 2. Powder X-ray diffraction data (d in Å) for hydroplumboelsmoreite.
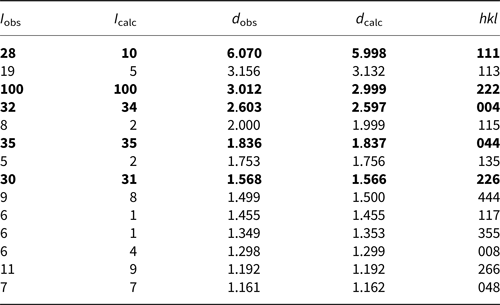
The strongest lines are given in bold.
Single-crystal X-ray diffraction and structure determination
The single-crystal X-ray analysis was carried out using a Rigaku Oxford Diffraction XtaLAB PRO-007HF rotating anode microfocus X-ray source (1.2 kW, MoKα, λ = 0.71073 Å) and a hybrid pixel array detector single-crystal diffractometer. The analytical conditions were 50 kV, 24 mA and an exposure time of 100 s per frame. A single crystal particle (10 μm × 10 μm × 10 μm in size) from the powder X-ray diffraction sample was analysed. The structure solution and refinement were performed using SHELX 2014 (Sheldrick, Reference Sheldrick2015), and the crystal structure was determined using the direct method. The details of the data collection and structure refinement for hydroplumboelsmoreite are summarised in Table 3. The atom coordinates and site occupancies are listed in Table 4. The anisotropic displacement parameters are listed in Table 5. Selected geometric parameters are listed in Table 6. The bond valences and bond-valence sums are presented in Table 7. The crystal structure of hydroplumboelsmoreite is shown in Fig. 6. The crystal structure refinement converged to R 1 = 0.0459 for 80 reflections with |F o| > 4σF and 0.0509 for all 94 reflections. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
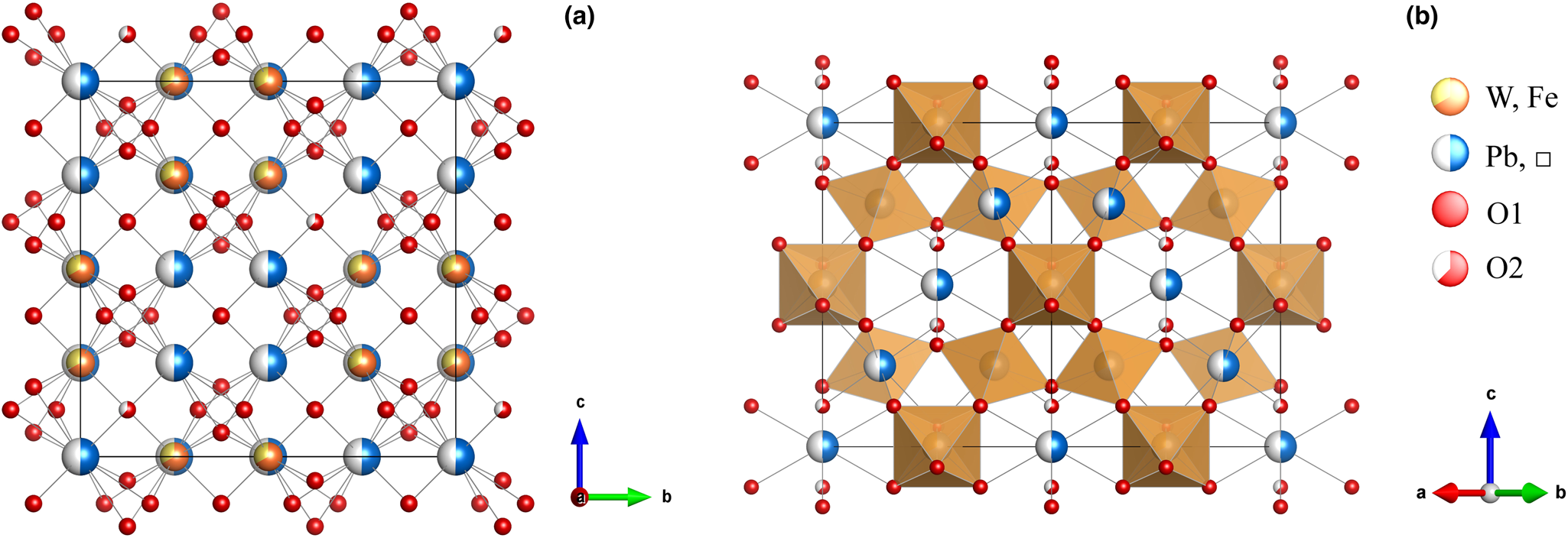
Fig. 6. General projection of the crystal structure of hydroplumboelsmoreite (a) along the a-axis and (b) the [110] projection. A site: (Pb,□). Drawn using Vesta (Momma and Izumi, Reference Momma and Izumi2011).
Table 3. Data collection and structure refinement details for hydroplumboelsmoreite.

*w = 1/[σ2(F 02) + (aP)2 + bP], where P = [max(F 02, 0) + 2Fc 2]/3
Table 4. Atomic coordinates for hydroplumboelsmoreite.
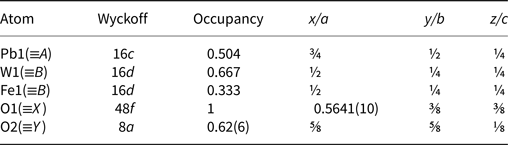
Table 5. Anisotropic displacement parameters and isotropic displacement parameters (Å2) for hydroplumboelsmoreite.
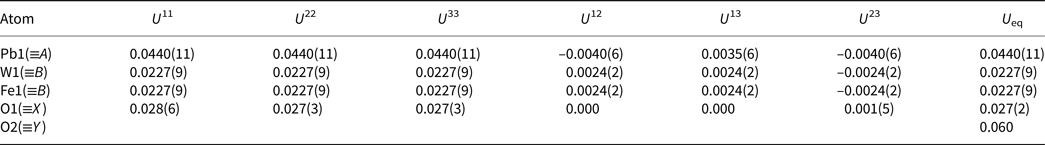
Table 6. Selected geometric bond distances (Å) for hydroplumboelsmoreite.

Table 7. Bond-valence sums for hydroplumboelsmoreite.

Note: The bond strength for the A site is based on an occupancy of 0.504Pb2+, a B site occupancy of 0.667W6+ + 0.333Fe3+, and an O2 occupancy of 0.626. The bond-valence calculations were done using the equation and constants of Brown (Reference Brown1977), S = exp[R 0 – d 0)/b], bond parameters for Pb2+–O from Krivovichev and Brown (Reference Krivovichev and Brown2001), bond parameters for W6+–O from Brese and O'Keeffe (Reference Brese and O'Keeffe1991), and bond parameters for Fe3+–O from Gagné and Hawthorne (Reference Gagne and Hawthorne2015).
The research results show that this mineral crystallises in the cubic system, space group Fd $\bar{3}$![]() m, with lattice parameters of a = 10.3377(5) Å, V = 1104.77(16) Å3 and Z = 8. The crystal structure is the pyrochlore type structure. W is [6]-coordinated (site 16c), is located at the centre of octahedron, and the W−O bond length is 1.944 Å. Pb, as a large cation, is [8]-coordinated (site 16d), is distributed in the channel, and the Pb−O bond length is 2.238 Å and 2.652 Å. Pb dominantly occupies the A site, while H2O predominantly occupies the Y site (site 8b). Thus, hydroplumboelsmoreite is still a pyrochlore-supergroup mineral. The ideal end-member formula is (Pb1□1)Σ2(W1.33Fe3+0.67)Σ2O6(H2O) based on the crystal structure.
m, with lattice parameters of a = 10.3377(5) Å, V = 1104.77(16) Å3 and Z = 8. The crystal structure is the pyrochlore type structure. W is [6]-coordinated (site 16c), is located at the centre of octahedron, and the W−O bond length is 1.944 Å. Pb, as a large cation, is [8]-coordinated (site 16d), is distributed in the channel, and the Pb−O bond length is 2.238 Å and 2.652 Å. Pb dominantly occupies the A site, while H2O predominantly occupies the Y site (site 8b). Thus, hydroplumboelsmoreite is still a pyrochlore-supergroup mineral. The ideal end-member formula is (Pb1□1)Σ2(W1.33Fe3+0.67)Σ2O6(H2O) based on the crystal structure.
The structure refinement process and water content analysis were as follows: The occupancy of the A and B sites were fixed based on the compositional data collected from the homologous particle. The A site was occupied predominantly by Pb; and the B site was occupied by W and Fe. As other members of the elsmoreite group, i.e. hydroxykenoelsmoreite (Mills et al., Reference Mills, Christy, Kampf, Birch and Kasatkin2017), are found in lower symmetric space groups due to the ordered distributions of Fe and W, we tried to find lower symmetric space groups to solve the structure of hydroplumboelsmoreite, however no satisfactory results were found. Hence, the W and Fe should be disordered in our sample. The B site was fixed with (W1.33Fe3+0.67) in the refinement process. The X site was assumed to be fully occupied, which was proven by the bond valence sums (BVS).
When we assumed the Y site (O2) was fully occupied by O# (e.g. O2–, OH–, or H2O), the U eq of O2 was 0.14766 Å2, which seemed too high for O atoms. This may have been due to the following two reasons: (1) the quality of the diffraction data was not satisfactory; and (2) the Y site was not fully occupied by O#. The first reason seems untenable, as there is another O1 atom (X site) in the structure with a lower U eq value of 0.027, and the R 1 and wR 2 values are acceptable (Table 8). As for the second reason, in pyrochlore-supergroup minerals (including the elsmoreite group), the Y site can be occupied by □, O2–, OH–, H2O, F–, K+, Cs+ and Rb+ (Atencio et al., Reference Atencio, Andrade, Christy, Giere and Kartashov2010). The electron microprobe analysis (EMPA) data shows that there are trace amounts of K, but no detectable F, Cs and Rb. Therefore, only □, O, OH–, or H2O is possible in the Y site of our sample.
Table 8. Site occupancy factors (s.o.f), R 1 and difference-Fourier values for the Y site under different U iso values.
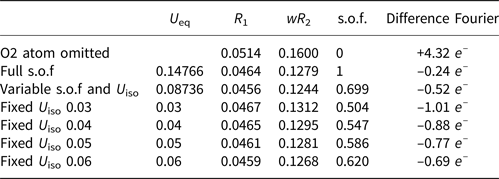
The nature of the occupancy of the Y site was determined using the successful refinement procedure described by Li et al. (Reference Li, Li, Fan, Fan and Nahdi2020) for hydroxyplumbopyrochlore. (1) Omitting the O2 atom from the refinement results in R 1 = 0.051 and the largest peak in the difference-Fourier map shows 4.32 e – at the Y site, suggesting an O# occupancy of >50%. (2) The site occupancy and displacement parameters for O2 were allowed to vary simultaneously, resulting in the site occupancy factor (s.o.f.) of O2 = 0.699 and U eq = 0.087. This seems too high for this sample. If there are H atoms around the O2, the higher U eq will contain part of the contributions of the H to the O2, leading to a higher s.o.f. value of the O2. (3) Based on the U eq of the O2 fixed in a range of reasonable U iso values, refinement results of the O2 occupancy were obtained as presented in Table 8. The U iso value of 0.06 Å2 in the Y site is acceptable in the structure refinement process of pyrochlore-supergroup minerals (Li et al., Reference Li, Li, Fan, Fan and Nahdi2020), and it produces a good result of O0.62□0.38, with lower R 1 and wR 2 values than under the fully occupied model. (4) The difference-Fourier electron density (DFED) along (111) (Fig. 7) shows that there are obvious positive anomalies around O2 (in blue) compared to the lack of an obvious positive anomaly around O1 in the X site, which is powerful evidence of the existence of H atoms around O2 (Plasil et al., Reference Plášil, Škoda, Fejfarová, Čejka, Kasatkin, Dušek, Talla, Lapčák, Machovič and Dini2014). The 3D−DFED around O2 (Fig. 8) shows that ten maxima in the additional electron density occur, four of them directed towards the face centres and six towards edge mid-points of the [O2Pb14] tetrahedron, away from the O2−Pb1 vectors; these are interpreted as possible locations of the H atoms. Angles between O−H vector directions such as [111]^[1$\bar{1}\bar{1}$![]() ] and [100]^[$\bar{1}$
] and [100]^[$\bar{1}$![]() 11] are 109.5° and 125.3°, respectively. These are broadly compatible with the 104° typical for an H2O molecule and suggest possible orientations for that molecule. The Raman spectrum and BVS calculation also demonstrate the existence of molecular H2O in the Y site. As the determination of the exact H positions using only the DFED is not reliable, we do not list the atomic coordinates of H. (5) Subsequently, (YO#0.62,□0.38) in the Y site is indicated, where O# denotes the amount of occupation needed to balance the formula's charge recalculated into O atoms. It should be noted that the empirical formula from the EMPA shows the requirement of a charge of –0.38 in the Y site, thus 0.43H2O, 0.19O2– and 0.38□ (vacancy) are assigned to the Y site. This corresponds to the 1.27 wt.% H2O chemical composition and 99.08 wt.% total. Therefore, the empirical formula calculated on the basis of B = 2 is (Pb1.05Sr0.05Ce3+0.07Na0.01□0.82)Σ2(W1.32Fe3+0.67Zr0.01)Σ2O6[(H2O)0.43O0.19□0.38]Σ1.
11] are 109.5° and 125.3°, respectively. These are broadly compatible with the 104° typical for an H2O molecule and suggest possible orientations for that molecule. The Raman spectrum and BVS calculation also demonstrate the existence of molecular H2O in the Y site. As the determination of the exact H positions using only the DFED is not reliable, we do not list the atomic coordinates of H. (5) Subsequently, (YO#0.62,□0.38) in the Y site is indicated, where O# denotes the amount of occupation needed to balance the formula's charge recalculated into O atoms. It should be noted that the empirical formula from the EMPA shows the requirement of a charge of –0.38 in the Y site, thus 0.43H2O, 0.19O2– and 0.38□ (vacancy) are assigned to the Y site. This corresponds to the 1.27 wt.% H2O chemical composition and 99.08 wt.% total. Therefore, the empirical formula calculated on the basis of B = 2 is (Pb1.05Sr0.05Ce3+0.07Na0.01□0.82)Σ2(W1.32Fe3+0.67Zr0.01)Σ2O6[(H2O)0.43O0.19□0.38]Σ1.
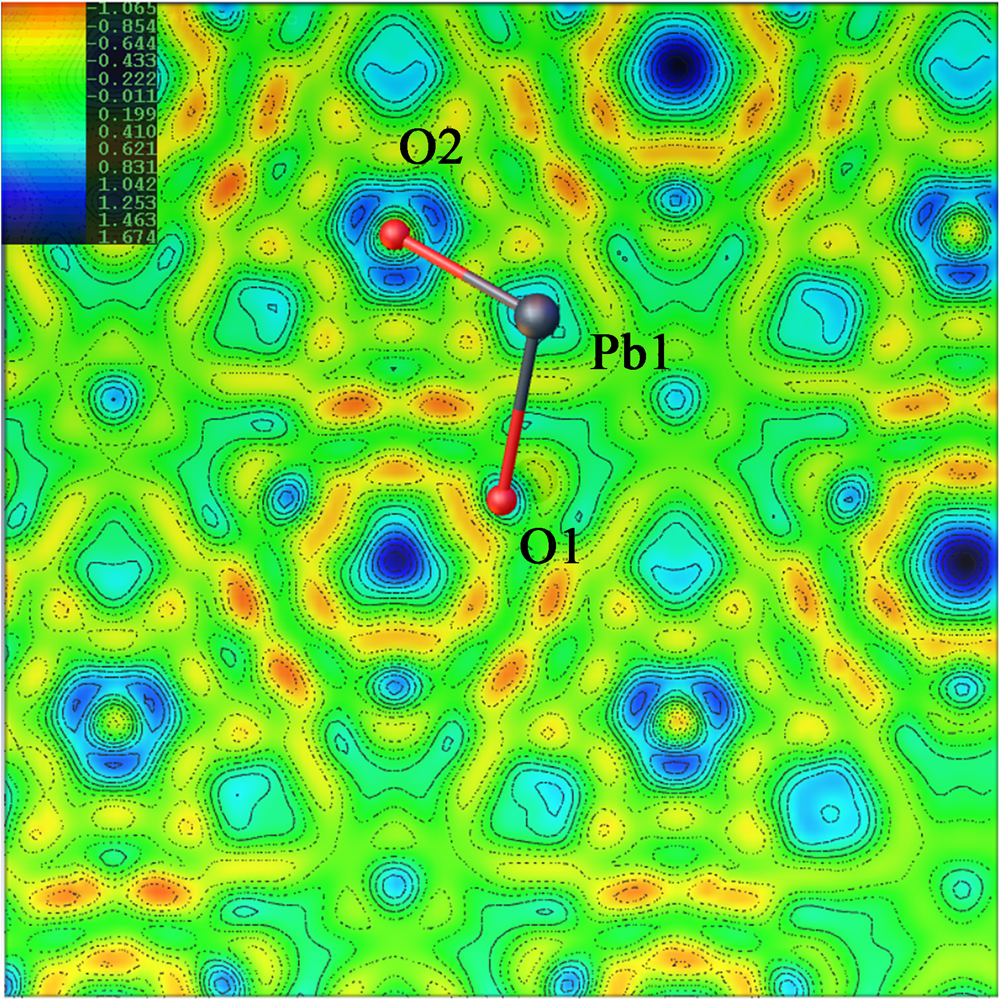
Fig. 7. Difference-Fourier electron density mapping along (111). Drawn using Olex2 (Dolomanov et al., Reference Dolomanov, Bourhis, Gildea, Howard and Puschmann2009).
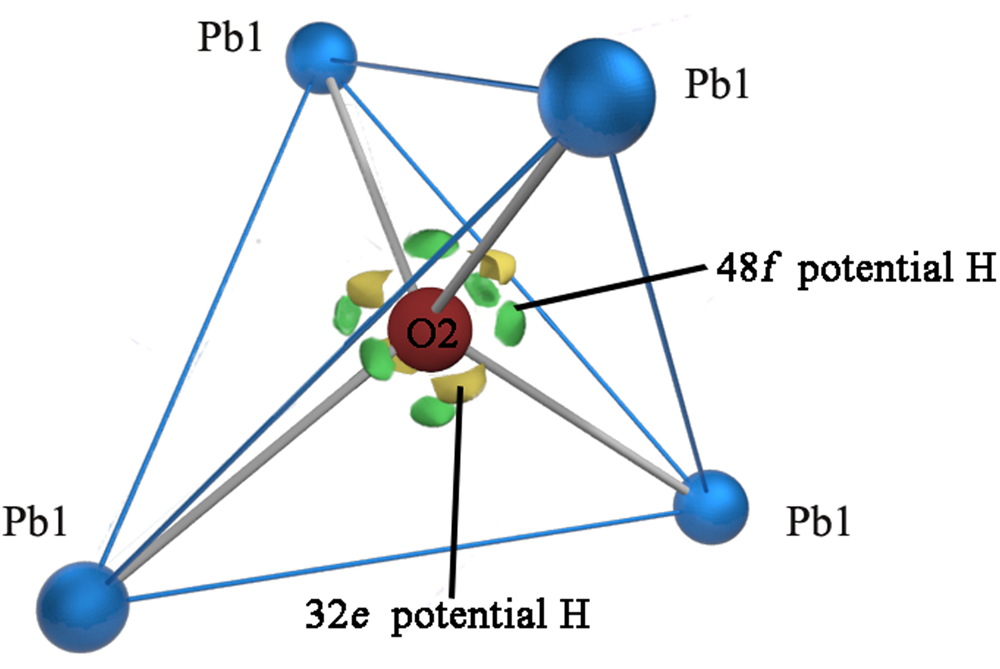
Fig. 8. Difference-Fourier electron density in the vicinity of the Pb1/O2 atoms. View of part of the hydroplumboelsmoreite structure, where the significant maxima of the positive difference electron density is located only around the O2 atom and four of them direct towards the face centres and six towards edge mid-points of the tetrahedron, away from the O2−Pb1 vectors.
In fact, for the full or open occupancies of the Y site, or a fixed U eq = 0.06, the refinements based on these three conditions lead to the Y site being occupied by [(H2O)0.81O0.19], [(H2O)0.51O0.19□0.30], or [(H2O)0.43O0.19□0.38], which all result in the conclusion that H2O is dominant in the Y site.
Therefore, this new mineral is an elsmoreite-group mineral of the pyrochlore supergroup, and it was named hydroplumboelsmoreite according to the predominance of Pb in the A site and molecular H2O in the Y site.
Discussion
Relationship to other mineral species
Hydroplumboelsmoreite is a new member of the pyrochlore supergroup. Its properties are compared with those of other members of the elsmoreite group in Table 9. A proposal for ‘hydroelsmoreite’ (IMA 2019-066) is being resubmitted to the CNMNC after revision. This phase is closely related to hydroplumboelsmoreite and was found in the same sample, but has obvious differences in chemical composition, crystal structure and hardness.
Table 9. Comparison of the properties of the related elsmoreite-group minerals.*
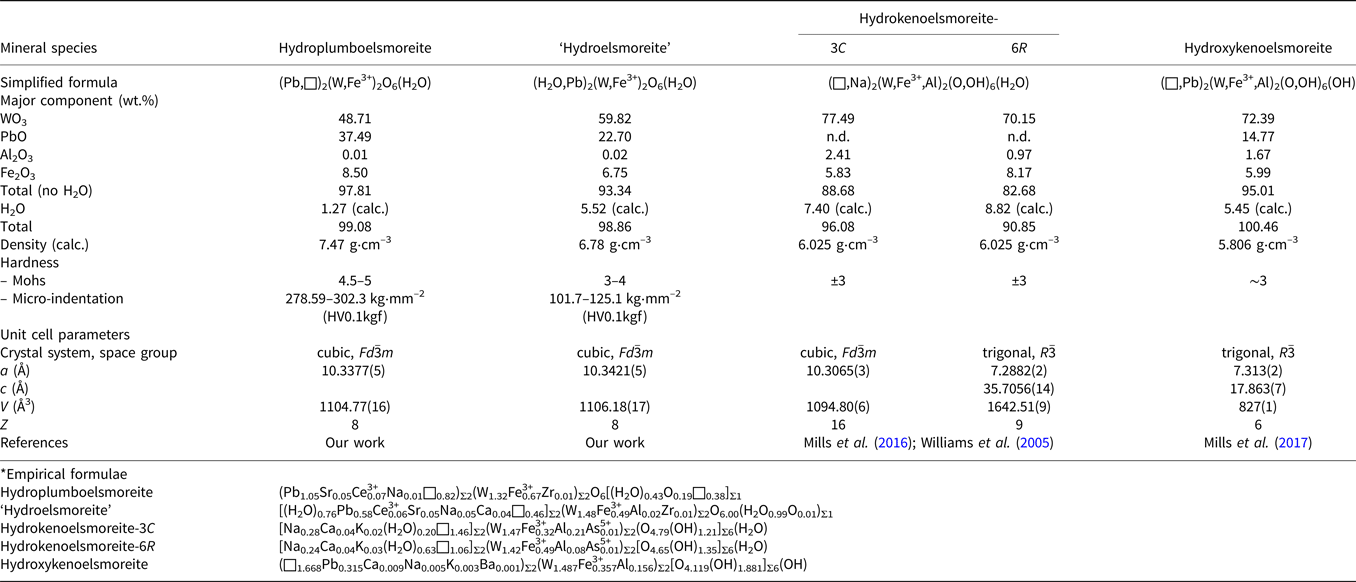
It should be noted that both of the previous two IMA-approved elsmoreite-group minerals, hydroxykenoelsmoreite (Mills et al., Reference Mills, Christy, Rumsey and Spratt2016) and hydrokenoelsmoreite (Mills et al., Reference Mills, Christy, Kampf, Birch and Kasatkin2017), have been reported to deviate from the ideal cubic system due to ordering of Na and (□,H2O) in the A site or due to Fe3+ in the B site. However, the ordered distributions in these two sites were not observed in the hydroplumboelsmoreite analysed in this study and implies that the full cubic symmetry of the ideal pyrochlore structure is maintained. This phenomenon deserves more attention in elsmoreite-group minerals.
Chemical composition evolution
In the ideal formula, (PbA 0)Σ2(W1.33Fe3+0.67)Σ2O6(H2O), which is charge balanced, the A site generally contains Pb2+ and other [8]-coordinated cations, and thus, the B site may contain subordinate Fe3+ or any other cations with lower charges to balance the charge. Neglecting minor substituents, the major components may have the following substitutions to make the formula electronically neutral: (PbxA 02–x)Σ2(W2–yFey)Σ2O6(H2O). In addition, there is a proportionality between the bivalence cations in the A site and the trivalent cations in the B site, where A 2+/B 3+ = 1.5 and x = 1.5y.
Thus, the electrically neutral end-member formula in the sense described by Hawthorne (Reference Hawthorne2002) can be written as follows: if y < 0.67 and x < 1, the corresponding end-member formula is (A 0)2W2O6(H2O) (labelled as F1 in Fig. 9), that is, ‘hydroelsmoreite’ or hydrokenoelsmoreite; otherwise, if 0.67 ≤ y < 1 and 1≤ x ≤ 2, the corresponding end-member formula is (PbA 0)Σ2(W1.33Fe3+0.67)Σ2O6(H2O) (labelled as F2 in Fig. 9) or (Pb1.5A 00.5)Σ2(WFe3+)Σ2O6(H2O) (labelled as F3 in Fig. 9), that is, hydroplumboelsmoreite. Hydroplumboelsmoreite and Pb-poor analogues such as ‘hydroelsmoreite’ and hydrokenoelsmoreite are named on the basis of dominant constituents of dominant valency groups in the Y, A and B sites in accord with Atencio et al. (Reference Atencio, Andrade, Christy, Giere and Kartashov2010). It should be noted that the end-member formula can be used as the ideal formula for hydroplumboelsmoreite is not unique although the former was found to be dominant in this study.
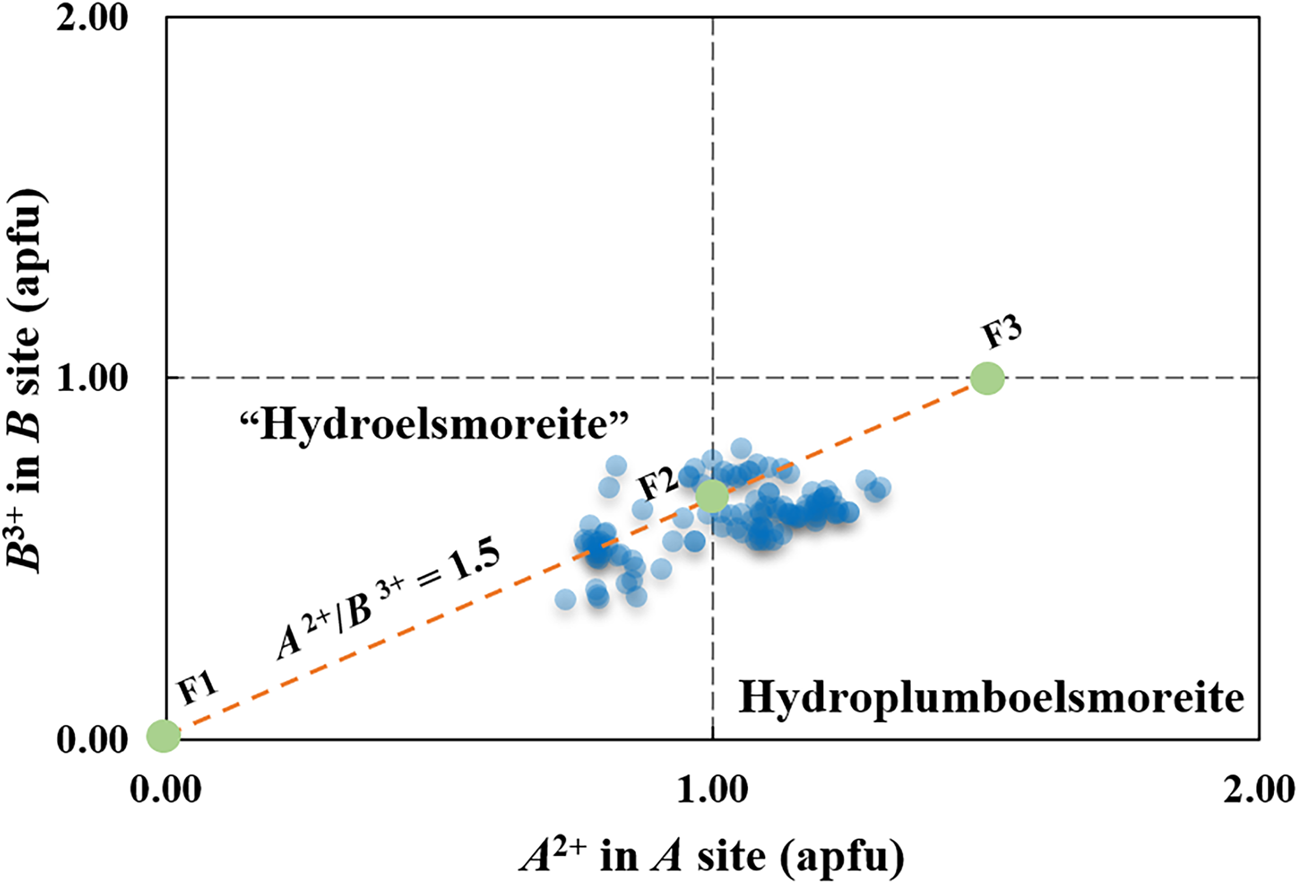
Fig. 9. Two-dimensional plots of the divalent A site cations and trivalent B site cations in hydroplumboelsmoreite and ‘hydroelsmoreite’. F1 = (A 0)2W2O6(H2O), F2 = (PbA 0)Σ2(W1.33Fe3+0.67)Σ2O6 (H2O), and F3 = (Pb1.5A 00.5)Σ2(WFe3+)Σ2O6(H2O).
Making the assumption of negligible substitutions other than A 0 for Pb-dominant A 2+ in the A site and Fe3+ for W6+ in the B site, 107 compositions obtained by WDS with polished fragments in the abovementioned epoxy block are plotted in Fig. 9. Although there is considerable solid solution that straddles the line between hydroplumboelsmoreite and ‘hydroelsmoreite’, the analysis points clearly cluster around the ideal composition F2 = (PbA 0)Σ2(W1.33Fe3+0.67)Σ2O6(H2O).
Given the large charge difference between W and Fe, the preference for a W:Fe ratio of 2:1 may imply short-range order of these cations in the B site that is not reflected in the long-range average structure.
Conclusions
It appears that the original ‘jixianite’ sample with which the average chemical data (Liu, Reference Liu1979) derived via wet-chemical analysis of powdered sample is a combination of raspite, hydroplumboelsmoreite and ‘hydroelsmoreite’.
The compositional and crystal structural data collected in this study for a hydroplumboelsmoreite crystal unequivocally indicate that structurally, it belongs to the pyrochlore supergroup. Thus, it should be classified as hydroplumboelsmoreite in accordance with the classification rules for pyrochlore-supergroup minerals. It more specifically belongs to the elsmoreite group (W6+ predominantly in the B site), with Pb predominantly in the A site and H2O predominantly in the Y site.
Acknowledgements
We acknowledge Prof. Ritsuro Miyawaki and Prof. Frédéric Hatert of the CNMNC and its members for their valuable suggestions on hydroplumboelsmoreite. A cotype specimen of ‘jixianite’ was provided by Liu Jianchang to whom we express our gratitude. We also thank Ge Xiangkun and Tai Zongyao for their kind assistance of chemical analyses. In addition, we acknowledge the efforts of Principal Editor Stuart Mills and of reviewer Prof. Peter Leverett, Mike Rumsey and an anonymous reviewer for their detailed reviews. We appreciate all the authors of hydroxyplumbopyrochlore for their inspiration to us. This work was supported financially by the National Natural Science Foundation of China (41672043) and the China National Postdoctoral Program for Innovative Talents (BX20190304).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2021.86