Published online by Cambridge University Press: 28 February 2018
The crystal structure of the mineral gortdrumite, a rare copper-mercury-iron sulfide, was solved using intensity data collected using a crystal from the Neuschurf adit, Leogang, Salzburg, Austria. This study revealed that the structure is triclinic, space group P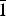 $\bar 1$, with cell parameters: a = 9.677(4), b = 9.865(5), c = 11.992(5) Å, α = 77.85(4), β = 79.42(3), γ = 76.30(4)°, V = 1076.5(8) Å3 and Z = 1. The refinement led to an R index of 0.0833 for 3335 independent reflections and 143 parameters. Twelve S sites (one with half occupancy) and eighteen metal sites (5 Hg, 12 Cu and 1 Fe) occur in the crystal structure of gortdrumite. Mercury cations link two sulfur atoms in a linear coordination, Cu cations are found in various low-coordination (2, 3 and 4) sites, in agreement with the Cu preference for such environments, and Fe in tetrahedral coordination. Metal–metal interactions are also present and these contacts are discussed in relation with other copper sulfides, intermetallics and pure metals. Electron microprobe analyses of the crystal used for the structural study led to the formula Cu24.83Fe1.73Hg9.09S22.35, on the basis of 58 atoms. On the basis of information gained from the structural and chemical characterization, the crystal-chemical formula was revised to Cu24Fe2Hg9S23 (Z = 1) instead of (Cu,Fe)6Hg2S5 as reported previously.
$\bar 1$, with cell parameters: a = 9.677(4), b = 9.865(5), c = 11.992(5) Å, α = 77.85(4), β = 79.42(3), γ = 76.30(4)°, V = 1076.5(8) Å3 and Z = 1. The refinement led to an R index of 0.0833 for 3335 independent reflections and 143 parameters. Twelve S sites (one with half occupancy) and eighteen metal sites (5 Hg, 12 Cu and 1 Fe) occur in the crystal structure of gortdrumite. Mercury cations link two sulfur atoms in a linear coordination, Cu cations are found in various low-coordination (2, 3 and 4) sites, in agreement with the Cu preference for such environments, and Fe in tetrahedral coordination. Metal–metal interactions are also present and these contacts are discussed in relation with other copper sulfides, intermetallics and pure metals. Electron microprobe analyses of the crystal used for the structural study led to the formula Cu24.83Fe1.73Hg9.09S22.35, on the basis of 58 atoms. On the basis of information gained from the structural and chemical characterization, the crystal-chemical formula was revised to Cu24Fe2Hg9S23 (Z = 1) instead of (Cu,Fe)6Hg2S5 as reported previously.
Associate Editor: Andrew Christy