Published online by Cambridge University Press: 05 July 2018
Domerockite, Cu4(AsO4)2(AsO3OH)(OH)3·H2O, is a new mineral from the Dome Rock Mine, South Australia. It occurs as aggregates of bluish green, equant to short prismatic and tabular crystals up to 0.3 mm long and 0.2 mm across. Domerockite is translucent, with a vitreous lustre and pale green streak. It displays no fluorescence under UV irradiation. The mineral is brittle with an uneven fracture, a Mohs hardness of ∼3 and a calculated density of 4.44 g/cm3 (based on the structure refinement). Optically, it is biaxial negative, with α = 1.798(4), β = 1.814(4), γ = 1.817(4), 2Vcalc. = 46°; pleochroism is very weak; X pale greenish yellow, Y greenish blue, Z greenish blue; absorption X < Y = Z; orientation is uncertain. Chemical analysis by electron microprobe gave CuO 52.04, ZnO 0.78, BaO 0.11, As2O537.67, P2O50.32, SiO20.24, H2O 8.84, total 100.00 wt.%, with H2O calculated by difference. The empirical chemical formula is (Cu3.94, Zn0.06)Σ4.00H0.91(As1.97, P0.03, Si0.02)Σ2.02O8(OH)3.00˙H2O based on 12 oxygen atoms.
Domerockite is triclinic, space group P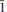 , with a = 5.378(11), b = 8.962(18) c = 9.841(2) Å, α = 75.25(3), β = 83.56(3), γ = 79.97(3)°, V = 450.5(16) Å3 and Z = 2. The eight strongest lines in the X-ray powder diffraction pattern are [d (Å), (I)(hkl)]: 4.716 (30)(101, 002, 111), 3.697 (25)(121), 3.605 (30)(120, 12
, with a = 5.378(11), b = 8.962(18) c = 9.841(2) Å, α = 75.25(3), β = 83.56(3), γ = 79.97(3)°, V = 450.5(16) Å3 and Z = 2. The eight strongest lines in the X-ray powder diffraction pattern are [d (Å), (I)(hkl)]: 4.716 (30)(101, 002, 111), 3.697 (25)(121), 3.605 (30)(120, 12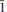 ), 3.119 (60)(12
), 3.119 (60)(12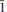 ), 3.073 (100)(1
), 3.073 (100)(1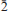
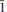 ), 2.856 (40)(02
), 2.856 (40)(02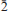 , 030), 2.464 (50)(212, 1
, 030), 2.464 (50)(212, 1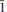 3), 2.443 (40)(014). The crystal structure of domerockite has been solved by direct methods and refined to an R index of 7.44% using 2635 observed reflections. The structure comprises [Cuφ4] (φ = O, OH) chains of edge-sharing sharing, distorted octahedra that extend along [10
3), 2.443 (40)(014). The crystal structure of domerockite has been solved by direct methods and refined to an R index of 7.44% using 2635 observed reflections. The structure comprises [Cuφ4] (φ = O, OH) chains of edge-sharing sharing, distorted octahedra that extend along [10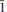 ] and are decorated by AsO4 tetrahedra to form sheets in the (010) plane. Dimers of edge-sharing [CuO4(OH)(H2O)] octahedra share corners with dimers of edge-sharing [CuO4(OH)] square pyramids to form zigzag chains which extend along [101]. The chains lie between and link to the sheets by sharings corners of octahedra, square pyramids and tetrahedra to form a heteropolyhedral framework.
] and are decorated by AsO4 tetrahedra to form sheets in the (010) plane. Dimers of edge-sharing [CuO4(OH)(H2O)] octahedra share corners with dimers of edge-sharing [CuO4(OH)] square pyramids to form zigzag chains which extend along [101]. The chains lie between and link to the sheets by sharings corners of octahedra, square pyramids and tetrahedra to form a heteropolyhedral framework.