Published online by Cambridge University Press: 07 June 2021
Microporous slicates with the pharmacosiderite structure and the general formula A3–xH1+x[Ti4O4(SiO4)3](H2O)n (A = Na, K, Cu), (n = 6–9, x = 0–2) are outstanding in their ion-exchange properties. The ivanyukite mineral group consists of three species, one of which has two polymorphs. The minerals forming a progressive series: ivanyukite-Na-T → ivanyukite-Na-C → ivanyukite-K → Cu-rich ivanyukite-K → ivanyukite-Cu, have been studied by single-crystal X-ray diffraction, electron microprobe analysis and Raman spectroscopy. The microporous heteropolyhedral framework of the ivanyukite-group minerals is based on cubane-like [Ti4O4]8+ clusters that share common corners with SiO4 tetrahedra to form wide three-dimensional channels suitable for the migration of Na+, K+ and Cu2+ ions. Ivanyukite-Na-T that has a R3m symmetry loses Na+ in aqueous solutions via the substitution Na+ + O2‒ ↔ □ + OH‒, which allows for the migration of K+ ions and transformation of initial structure into the cubic (P$\bar{4}3m$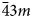 ) ivanyukite-Na-C polymorph or into ivanyukite-K, when most of Na is lost. Natural ivanyukite-Na-C is shown to contain domains of both R3m (subordinate) and P$\bar{4}3m$
) ivanyukite-Na-C polymorph or into ivanyukite-K, when most of Na is lost. Natural ivanyukite-Na-C is shown to contain domains of both R3m (subordinate) and P$\bar{4}3m$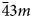 (dominant) symmetry with the chemical composition determining the stability and dominance of cubic or trigonal forms. Incorporation of Cu into the crystal structure ivanyukite-K via the substitution K+ + OH− ↔ Cu2+ + O2− in aqueous solutions results in the formation of ivanyukite-Cu. Post-crystallisation processes (such as exchange of Na+, K+, Cu2+, and/or hydration/dehydration of primary phases) are widespread in hyperagpaitic rocks of the Kola alkaline massif and the respective mineral transformations contribute to the diversity of mineral species.
(dominant) symmetry with the chemical composition determining the stability and dominance of cubic or trigonal forms. Incorporation of Cu into the crystal structure ivanyukite-K via the substitution K+ + OH− ↔ Cu2+ + O2− in aqueous solutions results in the formation of ivanyukite-Cu. Post-crystallisation processes (such as exchange of Na+, K+, Cu2+, and/or hydration/dehydration of primary phases) are widespread in hyperagpaitic rocks of the Kola alkaline massif and the respective mineral transformations contribute to the diversity of mineral species.
This paper is part of a thematic set ‘Alkaline Rocks’ in memory of Dr Gregory Yu. Ivanyuk
Associate Editor: Elena Zhitova