Published online by Cambridge University Press: 05 July 2018
The new mineral therasiaite, ideally (NH4)3KNa2Fe2+Fe3+(SO4)3Cl5, was found in a mediumtemperature (∼250°C) intracrater active fumarole at La Fossa crater, Vulcano, Aeolian Islands, Sicily, Italy. It occurs on a pyroclastic breccia as brown to dark brown equant to short prismatic crystals up to 0.1 mm in length, in association with salammoniac, kremersite and adranosite. The mineral is monoclinic, space group: Cc (no. 9) with a = 18.284(4), b = 12.073(2), c = 9.535(2) Å, β = 108.10(3)°, V = 2000.6(7) Å3 and Z = 4. The six strongest reflections in the X-ray powder diffraction pattern are: [dobs in Å(I)(hkl)] 2.812(100)( 23), 2.664(77)(
23), 2.664(77)(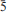 13), 3.297(28)(33
13), 3.297(28)(33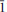 ), 3.208(14)(
), 3.208(14)(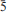
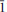 2), 3.008(12)(040), 2.942(11)(331). The empirical formula (based on 17 anions per formula unit (p.f.u.)) is (NH4)2.68K1.32Na2.04Fe1.76Al0.12Mn0.12S2.98O11.95Cl5.05. The measured density is 2.41(1) g cm−3, dcalc = 2.395 g cm−3. The mineral is biaxial (−) with α= 1.585(3) β = 1.615(3) and γ = 1.630(3) (white light). Using single-crystal diffraction data, the structure was refined to a final R(F) = 0.0240 for 5574 independent observed reflections [I > 2σ(I)]. The structure of therasiaite displays a novel topology and contains two independent, distorted octahedral Fe sites, with the Fe atoms in oxidation state 3+ and 2+, respectively, each surrounded by three Cl atoms and three oxygens of the sulfate ions. The Fe octahedra and the three independent sulfate anions are connected to form chains running along [001]. Voids between the chains are occupied by three independent ammonium ions (partially replaced by K+), one K+ and two Na+ ions. The formula resulting from the structure refinement is [(NH4)2.25K0.75]KNa2Fe2(SO4)3Cl5.
2), 3.008(12)(040), 2.942(11)(331). The empirical formula (based on 17 anions per formula unit (p.f.u.)) is (NH4)2.68K1.32Na2.04Fe1.76Al0.12Mn0.12S2.98O11.95Cl5.05. The measured density is 2.41(1) g cm−3, dcalc = 2.395 g cm−3. The mineral is biaxial (−) with α= 1.585(3) β = 1.615(3) and γ = 1.630(3) (white light). Using single-crystal diffraction data, the structure was refined to a final R(F) = 0.0240 for 5574 independent observed reflections [I > 2σ(I)]. The structure of therasiaite displays a novel topology and contains two independent, distorted octahedral Fe sites, with the Fe atoms in oxidation state 3+ and 2+, respectively, each surrounded by three Cl atoms and three oxygens of the sulfate ions. The Fe octahedra and the three independent sulfate anions are connected to form chains running along [001]. Voids between the chains are occupied by three independent ammonium ions (partially replaced by K+), one K+ and two Na+ ions. The formula resulting from the structure refinement is [(NH4)2.25K0.75]KNa2Fe2(SO4)3Cl5.