Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Schrauder, Marcus
and
Navon, Oded
1994.
Hydrous and carbonatitic mantle fluids in fibrous diamonds from Jwaneng, Botswana.
Geochimica et Cosmochimica Acta,
Vol. 58,
Issue. 2,
p.
761.
Wang, Alian
Dhamelincourt, P.
Meyer, H. O. A.
Guo, Lihe
and
Zhang, Andi
1994.
A carbon-rich multiphase inclusion in a Chinese diamond and its geochemical implications.
Contributions to Mineralogy and Petrology,
Vol. 117,
Issue. 1,
p.
15.
Kinny, Peter D.
and
Meyer, Henry O. A.
1994.
Zircon from the Mantle: A New Way to Date Old Diamonds.
The Journal of Geology,
Vol. 102,
Issue. 4,
p.
475.
Johnson, L.H.
Burgess, R.
Turner, G.
Milledge, H.J.
and
Harris, J.W.
2000.
Noble gas and halogen geochemistry of mantle fluids: comparison of African and Canadian diamonds.
Geochimica et Cosmochimica Acta,
Vol. 64,
Issue. 4,
p.
717.
SHIMOBAYASHI, Norimasa
and
KITAMURA, Masao
2001.
Growth habit of needle crystals in coats of coated diamonds..
Journal of Mineralogical and Petrological Sciences,
Vol. 96,
Issue. 5,
p.
188.
Burgess, R.
Layzelle, E.
Turner, G.
and
Harris, J.W.
2002.
Constraints on the age and halogen composition of mantle fluids in Siberian coated diamonds.
Earth and Planetary Science Letters,
Vol. 197,
Issue. 3-4,
p.
193.
Zedgenizov, D. A.
Kagi, H.
Shatsky, V. S.
and
Sobolev, N. V.
2004.
Carbonatitic melts in cuboid diamonds from Udachnaya kimberlite pipe (Yakutia): evidence from vibrational spectroscopy.
Mineralogical Magazine,
Vol. 68,
Issue. 1,
p.
61.
Izraeli, Elad S.
Harris, Jeffrey W.
and
Navon, Oded
2004.
Fluid and mineral inclusions in cloudy diamonds from Koffiefontein, South Africa.
Geochimica et Cosmochimica Acta,
Vol. 68,
Issue. 11,
p.
2561.
Tomlinson, Emma
De Schrijver, Isabel
De Corte, Katrien
Jones, Adrian P.
Moens, Luc
and
Vanhaecke, Frank
2005.
Trace element compositions of submicroscopic inclusions in coated diamond: A tool for understanding diamond petrogenesis.
Geochimica et Cosmochimica Acta,
Vol. 69,
Issue. 19,
p.
4719.
Shatsky, V. S.
Pal'yanov, Y. N.
Sokol, A. G.
Tomilenko, A. A.
and
Sobolev, N. V.
2005.
Diamond Formation in UHP Dolomite Marbles and Garnet-Pyroxene Rocks of the Kokchetav Massif, Northern Kazakhstan: Natural and Experimental Evidence.
International Geology Review,
Vol. 47,
Issue. 10,
p.
999.
Fritsch, Emmanuel
Moore, Moreton
Rondeau, Benjamin
and
Waggett, Richard G.
2005.
X-ray topography of a natural twinned diamond of unusual pseudo-tetrahedral morphology.
Journal of Crystal Growth,
Vol. 280,
Issue. 1-2,
p.
279.
Tomlinson, E.L.
Jones, A.P.
and
Harris, J.W.
2006.
Co-existing fluid and silicate inclusions in mantle diamond.
Earth and Planetary Science Letters,
Vol. 250,
Issue. 3-4,
p.
581.
Tomlinson, Emma L.
McMillan, Paul F.
Zhang, Ming
Jones, Adrian P.
and
Redfern, Simon A.T.
2007.
Quartz-bearing C–O–H fluid inclusions diamond: Retracing the pressure–temperature path in the mantle using calibrated high temperature IR spectroscopy.
Geochimica et Cosmochimica Acta,
Vol. 71,
Issue. 24,
p.
6030.
Zedgenizov, D.A.
Rege, S.
Griffin, W.L.
Kagi, H.
and
Shatsky, V.S.
2007.
Composition of trapped fluids in cuboid fibrous diamonds from the Udachnaya kimberlite: LAM-ICPMS analysis.
Chemical Geology,
Vol. 240,
Issue. 1-2,
p.
151.
Klein-BenDavid, Ofra
Izraeli, Elad S.
Hauri, Erik
and
Navon, Oded
2007.
Fluid inclusions in diamonds from the Diavik mine, Canada and the evolution of diamond-forming fluids.
Geochimica et Cosmochimica Acta,
Vol. 71,
Issue. 3,
p.
723.
Klein-BenDavid, Ofra
Wirth, Richard
and
Navon, Oded
2007.
Micrometer-scale cavities in fibrous and cloudy diamonds — A glance into diamond dissolution events.
Earth and Planetary Science Letters,
Vol. 264,
Issue. 1-2,
p.
89.
Rege, Sonal
Griffin, W.L.
Kurat, G.
Jackson, S.E.
Pearson, N.J.
and
O'Reilly, Suzanne Y.
2008.
Trace-element geochemistry of diamondite: Crystallisation of diamond from kimberlite–carbonatite melts.
Lithos,
Vol. 106,
Issue. 1-2,
p.
39.
Klein-BenDavid, Ofra
Logvinova, Alla M.
Schrauder, Marcus
Spetius, Zladislav V.
Weiss, Yaakov
Hauri, Erik H.
Kaminsky, Felix V.
Sobolev, Nikolay V.
and
Navon, Oded
2009.
High-Mg carbonatitic microinclusions in some Yakutian diamonds—a new type of diamond-forming fluid.
Lithos,
Vol. 112,
Issue. ,
p.
648.
Kvasnytsya, Victor M.
and
Wirth, Richard
2009.
Nanoinclusions in microdiamonds from Neogenic sands of the Ukraine (Samotkan’ placer): A TEM study.
Lithos,
Vol. 113,
Issue. 3-4,
p.
454.
Sobolev, N.V.
Logvinova, A.M.
and
Efimova, E.S.
2009.
Syngenetic phlogopite inclusions in kimberlite-hosted diamonds: implications for role of volatiles in diamond formation.
Russian Geology and Geophysics,
Vol. 50,
Issue. 12,
p.
1234.
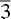 , corresponding to the dolomite structure, was positively identified. These crystals are classed as ankerites. Representative Mg:Fe:Ca ratios in the ankerites are 28 : 18 : 54, with cell dimensions a = 4.87 Å, c/a = 3.37. The ankerites contained small amounts of Ba, typically 1-3%, and smaller amounts of Sr, typically 0.2-2%. Three out of the 15 specimens contained >10% Ba. In two of these, Ba and Ca concentrations were roughly equal, and in one of the two, which had cell parameters a = 5.11 Å, c/a = 3.50, the space group symmetry R
, corresponding to the dolomite structure, was positively identified. These crystals are classed as ankerites. Representative Mg:Fe:Ca ratios in the ankerites are 28 : 18 : 54, with cell dimensions a = 4.87 Å, c/a = 3.37. The ankerites contained small amounts of Ba, typically 1-3%, and smaller amounts of Sr, typically 0.2-2%. Three out of the 15 specimens contained >10% Ba. In two of these, Ba and Ca concentrations were roughly equal, and in one of the two, which had cell parameters a = 5.11 Å, c/a = 3.50, the space group symmetry R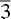 c, corresponding to the calcite structure, was verified.
c, corresponding to the calcite structure, was verified.

