Published online by Cambridge University Press: 31 March 2021
The new högbomite-group mineral magnesiohögbomite-6N12S, ideally Mg5Al11TiO23(OH), was found in calcite “vein-dikes” at the DeWitts Corners occurrence, lots 10 and 11, concession 1, Bathurst Township, Ontario, Canada. It forms tabular and short-prismatic crystals up to 5 mm in size. The major forms are pinacoid {0001} and hexagonal pyramid {11${\bar 2}$ 1}, sometimes modified by hexagonal prism {11${\bar 2}$
1}, sometimes modified by hexagonal prism {11${\bar 2}$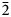 0}. The associated minerals are magnesiohögbomite-2N3S, spinel, corundum, diopside, magnesio-hastingsite, pargasite, clinochlore and calcite. Magnesiohögbomite-6N12S is dark brown to black with brown streak and vitreous lustre. It has no cleavage and its fracture is uneven. The Mohs hardness is 6½. Dcalc is 3.87 g/cm3. The infrared spectrum is reported. The composition (wt.%) is MgO 13.09, ZnO 0.46, FeO 11.91, Fe2O3 6.84, Al2O3 62.70, TiO2 4.44, H2O 0.99, total 100.43. The empirical formula calculated on the basis of 17 cations, excluding H+, is (Mg2.95Fe2+1.51Al0.49Zn0.05)Σ5(Al10.71Fe3+0.78Ti0.51)Σ12O23(OH). The simplified formula is (Mg,Fe)5(Al,Fe,Ti)12O23(OH). The mineral is trigonal, R${\bar 3}$
0}. The associated minerals are magnesiohögbomite-2N3S, spinel, corundum, diopside, magnesio-hastingsite, pargasite, clinochlore and calcite. Magnesiohögbomite-6N12S is dark brown to black with brown streak and vitreous lustre. It has no cleavage and its fracture is uneven. The Mohs hardness is 6½. Dcalc is 3.87 g/cm3. The infrared spectrum is reported. The composition (wt.%) is MgO 13.09, ZnO 0.46, FeO 11.91, Fe2O3 6.84, Al2O3 62.70, TiO2 4.44, H2O 0.99, total 100.43. The empirical formula calculated on the basis of 17 cations, excluding H+, is (Mg2.95Fe2+1.51Al0.49Zn0.05)Σ5(Al10.71Fe3+0.78Ti0.51)Σ12O23(OH). The simplified formula is (Mg,Fe)5(Al,Fe,Ti)12O23(OH). The mineral is trigonal, R${\bar 3}$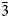 m, a = 5.7194(2), c = 83.069(5) Å, V = 2353.3(2) Å3 and Z = 6. The strongest reflections of the powder X-ray diffraction pattern [d,Å(I)(hkl)] are: 2.921(26)(0.1.23), 2.863(49)(110), 2.687(29)(0.1.26), 2.547(31)(0.1.$\overline{28}$
m, a = 5.7194(2), c = 83.069(5) Å, V = 2353.3(2) Å3 and Z = 6. The strongest reflections of the powder X-ray diffraction pattern [d,Å(I)(hkl)] are: 2.921(26)(0.1.23), 2.863(49)(110), 2.687(29)(0.1.26), 2.547(31)(0.1.$\overline{28}$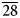 ) and 2.434(100)(1.1.18). The crystal structure was solved and refined from single-crystal X-ray diffraction data to R1 = 0.022. It is composed of alternating spinel (S) and nolanite (N) modules in the sequence 3 × (NSSNSS). The sequence of cubic ‘c’ and hexagonal ‘h’ closed-packed oxygen layers is 3 × (cccccchcccch). It is the first polysome in the högbomite supergroup with such a sequence.
) and 2.434(100)(1.1.18). The crystal structure was solved and refined from single-crystal X-ray diffraction data to R1 = 0.022. It is composed of alternating spinel (S) and nolanite (N) modules in the sequence 3 × (NSSNSS). The sequence of cubic ‘c’ and hexagonal ‘h’ closed-packed oxygen layers is 3 × (cccccchcccch). It is the first polysome in the högbomite supergroup with such a sequence.
Associate Editor: Charles A Geiger