Article contents
Bi-Sb energetics in sulfosalts and sulfides
Published online by Cambridge University Press: 05 July 2018
Abstract
Experimental brackets (300–450°C) on Sb-Bi partitioning between stibnite-bismuthinites (Sb,Bi)2S3 and sulfosalts in the AgSbS2–AgBiS2 binary subsystem (α-Ag(Sb,Bi)S2, β-Ag5(Sb,Bi)4I(Sb,Bi)IIS10) and extant constraints are used to define mixing properties and standard state Gibbs energies of Sb-Bi exchange reactions. They are also used to construct a phase diagram for Ag(Sb,Bi)S2 sulfosalts. We infer that the non-ideality associated with Sb-Bi mixing is largest in minerals of the β-Ag5(Sb,Bi)4I(Sb,Bi)IIS10 series, and is sufficient to produce miscibility gaps between an ordered intermediate species Ag5(Sb)4I(Bi)IIS10 and Sb- and Bi-end-members at T < 240°C (measured in terms of symmetric regular-solution-type parameters ¼WBi−SbIβ = WBi−SbIIβ ∼ 8.5 kJ/gfw). The non-ideality associated with the Sb-Bi substitution in stibnite-bismuthinite and α-Ag(Sb,Bi)S2 is ≈ 70% that in the Ag5(Sb,Bi)4I(Sb,Bi)IIS10 series (WBi−SbBS ≈ 12.0 kJ/gfw; WBi−Sbα ≈ 6.0 kJ/gfw). It is insufficient to produce exsolution at temperatures of ore deposition (T > Tc ≈ 88°C), but most likely is responsible for a preponderance in molar Sb/Bi ratios towards end-member compositions. Finally, positive Gibbs energies of the Sb-Bi exchange reactions and 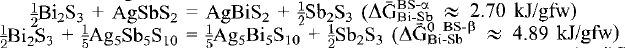 indicate that Bi is more compatible in stibnite-bismuthinite sulfides than in Ag(Sb,Bi)S2 sulfosalts.
indicate that Bi is more compatible in stibnite-bismuthinite sulfides than in Ag(Sb,Bi)S2 sulfosalts.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1999
References
- 9
- Cited by


