Note on Pauli's Exclusion Principle
Published online by Cambridge University Press: 24 October 2008
Extract
A simple explanation of Pauli's principle was first given with the wave mechanics. Its interpretation in the new theory was that the wave functions of Schrödinger were antisymmetrical in all the electrons concerned. Thus when the interactions of the electrons may be neglected, the wave function (for a system of n electrons) can never be of the form
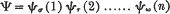
in nature, but only of the form
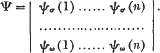
Hence σ, τ,…ω must be all distinct.
- Type
- Research Article
- Information
- Mathematical Proceedings of the Cambridge Philosophical Society , Volume 24 , Issue 3 , July 1928 , pp. 445 - 446
- Copyright
- Copyright © Cambridge Philosophical Society 1928
References
* See Fowler, , Proc. Roy. Soc. A, 113, p. 432 (1926).CrossRefGoogle Scholar
* See Hund, , Zeits. f. Phys. 43, p. 788 (1927).CrossRefGoogle Scholar
- 1
- Cited by


