Introduction
The Hawaiian lichen biota has generally been presumed to contain mostly widespread species, with a comparatively low degree of endemism at around 20–30% (Smith Reference Smith1993, Reference Smith2013; Eldredge & Miller Reference Eldredge and Miller1995). In contrast, endemism in vascular plants is c. 80% (Wagner et al. Reference Wagner, Herbst and Sohmer1999; Evenhuis & Eldredge Reference Evenhuis and Eldredge2002; Wagner & Herbst Reference Wagner and Herbst2002). However, recent molecular phylogenetic approaches indicate that many of the presumably widespread taxa in Hawaii represent distinct, putatively endemic species. Thus, in Lobariella and Pseudocyphellaria, revised phylogenetic classifications suggest putative endemism to be around 75% (Moncada et al. Reference Moncada, Reidy and Lücking2014a; Lücking et al. Reference Lücking, Moncada and Smith2017a).
The genus Sticta is the largest genus in Peltigeraceae subfamily Lobarioideae, with 200 species currently accepted and many more recognized on the basis of molecular phylogenetic studies (Moncada et al. Reference Moncada, Reidy and Lücking2014a, Reference Moncada, Lücking and Lumbsch2020; Lücking et al. Reference Lücking, Hodkinson and Leavitt2017b; Simon et al. Reference Simon, Goffinet, Magain and Sérusiaux2018). The most recent checklist of Hawaiian lichens (Smith Reference Smith2013) listed 11 names under Sticta. One of these, S. crocatoides f. sandwicensis Zahlbr., belongs in the genus Pseudocyphellaria (Moncada et al. Reference Moncada, Lücking and Suárez2014b). The ten remaining names included seven at species level and three at the level of variety, viz. S. ambavillaria (Bory) Ach., S. cyphellulata (Müll. Arg.) Hue, S. filix (Sw.) Nyl., S. fuliginosa (Dicks.) Ach., S. plumbicolor (Zahlbr.) Zahlbr., S. tomentosa (Sw.) Ach., S. weigelii (Ach.) Vain., S. weigelii var. beauvoisii (Delise) Hue, S. weigelii var. lutescens (Taylor) H. Magn. and S. weigelii var. peruviana (Delise) Vain. Sticta beauvoisii Delise was recently reinstated at species rank (McDonald et al. Reference McDonald, Miadlikowska and Lutzoni2003; Galloway Reference Galloway2006), whereas the correct application of the names S. weigelii var. lutescens and S. weigelii var. peruviana is unclear (Moncada et al. Reference Moncada, Lücking and Lumbsch2020). Thus, prior to molecular studies, the genus Sticta was represented in Hawaii by eight species, with only one, S. plumbicolor, putatively endemic, resulting in an inferred endemism of 13%. However, a recent phylogenetic revision distinguished 13 species, including seven new to science and retaining only three previously reported taxa, S. fuliginosa, S. plumbicolor and S. tomentosa, thus raising the estimate of endemism to 69% (Moncada et al. Reference Moncada, Lücking and Lumbsch2020).
In the current study, we present an updated taxonomic treatment of the genus Sticta in the Hawaiian archipelago based on these results, including a revision of numerous collections, diagnostic descriptions and illustrations, notes on the distribution and ecology of the species, and an identification key.
Material and Methods
The underlying revised taxonomy of Hawaiian Sticta was based on a recent phylogenetic revision, which revealed recently collected Hawaiian material as belonging to 10 clades representing 13 species (Moncada et al. Reference Moncada, Lücking and Lumbsch2020). Based on these results, we also revised herbarium collections mostly at HAW but also at some other herbaria (DUKE, F, NY, US) and in the Lichen Portal of the Consortium of North American Lichen Herbaria (https://lichenportal.org/cnalh), to assign specimens to the newly recognized taxa.
Morphological and anatomical details of the specimens were studied using a LEICA Zoom 2000 dissecting microscope and a ZEISS Axioskop compound microscope. We employed thin-layer chromatography (TLC) according to Orange et al. (Reference Orange, James and White2010).
Taxonomic Treatment
Key to species of Sticta in Hawaii
1 Vegetative propagules or marginal lobules absent; apothecia present; underside whitish, with thin tomentum and minute cyphellae; Kauai, Oahu, Molokai, Maui, Hawaii (Fig. 1A–D) Sticta tomentosa
Vegetative propagules or marginal lobules present; apothecia present or absent; underside variable2
2(1) Vegetative propagules soredia, predominantly marginal, white-grey; lobes broadly rounded; Kauai, Maui (Fig. 1E–H) Sticta limbata
Vegetative propagules isidia or phyllidia, or marginal lobules present, concolorous with the thallus or darker; lobes variable 3
3(2) Vegetative propagules predominantly laminal isidia, regularly dispersed over the entire lobe surface; lobes more or less broadly rounded4
Vegetative propagules predominantly marginal isidia, phyllidia, or with marginal lobules, if also laminal then clustered and leaving larger surface areas free; lobes irregularly truncate to elongate 6
4(3) Thallus extremely small (often only to 0.5 cm), pedunculate; cyphellae absent; Oahu (Fig. 1I–L) Sticta acyphellata
Thallus small to robust (1–5 cm diam.), not pedunculate; cyphellae present 5
5(4) Thallus small (c. 1 cm diam.); lobes with conspicuous marginal, white cilia; cyphellae very small (to 0.2 mm diam.), rounded; Kauai, Oahu, Maui (Fig. 1M–P) Sticta hawaiiensis
Thallus robust (to 5 cm diam.); lobes lacking distinct cilia; cyphellae becoming large (1–3mm diam.) and irregularly angulate; Kauai, Oahu, Maui, Hawaii (Fig. 1Q–T) Sticta fuliginosa
6(3) Thallus with somewhat large, strictly marginal lobules; apothecia rather frequent; lower tomentum rather thick but abruptly ending and leaving bare lobe margins; Kauai, Maui (Fig. 1U–X) Sticta antoniana
Thallus with small, predominantly marginal but sometimes also laminal isidia or phyllidia; apothecia rare or absent 7
7(6) Thallus very small (c. 1 cm), distinctly pedunculate, consisting of sparsely branched lobes with a basal stipe ascending from the substratum 8
Thallus small to robust (1–10 cm diam.), not pedunculate, with much branched lobes, overgrowing the substratum more or less horizontally 9
8(7) Cyphellae becoming rather large (1–3 mm diam.) and irregular; Kauai, Maui (Fig. 2A–D) Sticta flynnii
Cyphellae remaining small (0.2–0.5 mm diam.) and rounded; Oahu (Fig. 2E–H) Sticta smithii
9(7) Thallus remaining small (1–3 cm diam.); lobes with distinct black, marginal cilia; Kauai, Maui (Fig. 2I–L) Sticta emmanueliana
Thallus becoming robust (3–10 cm diam.); lobes lacking or with indistinct or whitish cilia 10
10(9) Thallus bluish grey when fresh, bluish to yellowish grey when dry, with conspicuous phyllidia; underside whitish, with small, inconspicuous cyphellae; scattered apothecia usually present; Kauai, Oahu, Molokai, Maui, Hawaii (Fig. 2M–P) Sticta plumbicolor
Thallus olive green to grey-brown when fresh, brownish when dry, with cylindrical to flattened isidia or small phyllidia; underside light to dark brown, with conspicuous cyphellae contrasting with the darker tomentum 11
11(10) Lower tomentum marginally pale; vegetative propagules (phyllidia) marginal and laminal (then clustered), concolorous with thallus; lobe surface often thinly scabrous, particularly towards the tips, and also often shallowly scrobiculate-foveolate; Kauai, Oahu, Molokai, Maui, Hawaii (Fig. 2Q–T) Sticta scabrosa subsp. hawaiiensis
Lower tomentum uniformly dark; vegetative propagules (isidia or phyllidia) predominantly marginal, cylindrical, darker than the thallus; lobe surface glabrous, even to uneven 12
12(11) Usually on soil between bryophytes, rarely epiphytic over bryophyte mats; lobes 4–7 mm broad; with cylindrical to somewhat flattened isidia; rhizines conspicuous; Maui, Hawaii (Fig. 2U–V) Sticta waikamoi
Usually epiphytic on branches, stems or tree trunks; lobes 7–15 mm broad; mostly with flattened and dorsiventral phyllidia; rhizines short; Kauai, Oahu, Maui, Hawaii (Fig. 2W–X) Sticta andina

Fig. 1. Habitus of Hawaiian Sticta. A–D, S. tomentosa. E–H, S. limbata. I–L, S. acyphellata. M–P, S. hawaiiensis. Q–T, S. fuliginosa. U–X, S. antoniana. Most of these images were taken in the field, therefore no scales are added. Sticta antoniana, S. fuliginosa, S. limbata and S. tomentosa are conspicuous species, whereas S. acyphellata and S. hawaiiensis are comparatively small, their individual thalli usually not exceeding 1 cm. In colour online.
The species
Sticta acyphellata Moncada & Lücking
Moncada et al., Pl. Fung. Syst. 65, 106 (2020); type: USA, Hawaii, Oahu, Koolau Range, Manoa Valley, 21°19ʹ55ʺN, 157°48ʹ43ʺW, 410–575 m, 2013, B. Moncada, R. Lücking & C. W. Smith 6923 (F—holotype!; B, HAW—isotypes!).
Primary photobiont cyanobacterial (Nostoc). Stipe present, short. Thallus monophyllous, individual thalli irregularly arranged, delicate, irregularly branched and dissected; lobes to 5 mm broad, spathuliform to truncate, ascending, not usually overlapping, plane to involute, their margins becoming dissected. Upper surface even, dark bluish grey when fresh, bluish grey when dry, glabrous, without papillae or pruina, with white maculae forming a reticulate pattern. Isidia frequent, marginal and laminal, 0.1–0.2 mm long and 0.05–0.1 mm broad, dark grey, shiny, flattened and somewhat imbricate, becoming somewhat arbuscular, branched and coralloid; arbusculae to 0.2 mm long and broad, distinctly flattened, their base lacking cyphellae, concolorous with the thallus. Lower surface uneven, forming vein-like ridges, minutely scrobiculate-foveolate between the ridges, white to cream. Primary tomentum dense but short and inconspicuous, whitish; hairs 20–70 μm long, composed of single, mostly unbranched, cylindrical, colourless hyphae. Secondary tomentum absent. Cyphellae absent.
Apothecia not observed.

Fig. 2. Habitus of Hawaiian Sticta. A–D, S. flynnii. E–H, S. smithii. I–L, S. emmanueliana. M–P, S. plumbicolor. Q–T, S. scabrosa subsp. hawaiiensis. U–V, S. waikamoi. W–X, S. andina. Most of these images were taken in the field, therefore no scales are added. Sticta andina, S. fuliginosa, S. plumbicolor, S. scabrosa subsp. hawaiiensis and S. waikamoi are conspicuous to large taxa, whereas S. flynnii and S. smithii are comparatively small, their individual thalli usually not exceeding 1 cm; S. emmanueliana is intermediate in size. In colour online.
Secondary chemistry
No substances detected by TLC; medulla K−.
Distribution and ecology
This species is thus far known only from the island of Oahu, probably overlooked elsewhere. It is difficult to detect in the field due to its diminutive size. It is found in liverwort carpets, such as Odontoschisma spp., on the lower trunk and exposed roots, generally no higher than 1.5 m above ground, in open, non-native, mature rainforest understory at lower elevations (410–575 m). The only identified phorophytes to date are Elaeocarpus bifidus and Cordyline fruticosa (as C. terminalis).
Remarks
This species is unique within the genus due to its diminutive size and the complete lack of cyphellae. It provides a contrary example of what is seen in Lobaria anomala (Brodo & Ahti) T. Sprib. & McCune and L. anthraspis (Ach.) T. Sprib. & McCune. Although producing pseudocyphellae, these two species form part of Lobaria s. str., which otherwise lacks lower surface pores (McCune et al. Reference McCune, Rosentreter, Spribille, Breuss and Wheeler2014; Cornejo & Scheidegger Reference Cornejo and Scheidegger2015).
Additional specimens examined
USA: Hawaii: Oahu, Koolau Mountains, Tantalus, 467 m, 1977, G. Y. Daida 503 (HAW); Oahu, Koolau Range, Manoa Valley, Manoa Cliffs Trail, 21°19ʹ55ʺN, 157°48ʹ43ʺW, 410–575 m, 2013, B. Moncada, R. Lücking & C. W. Smith 6918b (B), 6920 (B, F).
Sticta andina Moncada, Lücking & Sérusiaux
Moncada et al., Willdenowia (in press); type: Colombia, Bogotá, D.C., Sendero Peña Blanca, 04°26ʹ08ʺ to 04°26ʹ19ʺN, 74°08ʹ36ʺ to 74°08ʹ37ʺW, 2840–2860 m, 2015, R. Lücking (with C. Vargas) 39464 (B—holotype!; JBB—isotype!).
Primary photobiont cyanobacterial (Nostoc). Stipe absent. Thallus monophyllous, forming suborbicular rosettes or becoming irregular, to 15 cm across, mostly anisotomously branched; lobes 7–15 mm broad, elongate to flabellate, ±horizontal, adjacent to imbricate, involute to slightly canaliculate, their margins entire to sinuose or shallowly crenate. Upper surface smooth to rugose or shallowly scrobiculate, olive when fresh, brownish when dry, glabrous, with or without scattered papillae, without pruina, with indistinct, cream maculae; marginal cilia not differentiated but lower tomentum sometimes projecting beyond the margins to resemble short, brown-black, agglutinate to fasciculate cilia. Phyllidia and flattened isidia present, predominantly marginal, to 1 mm long and broad, rather dark grey-brown, somewhat shiny, branched and becoming coralloid to palmate with a basal stipe with diminutive cyphellae. Lower surface uneven to undulate, dark brown to blackish. Primary tomentum thick and dense to the lobe margins, spongy, forming dark brown to blackish brown, arachnoid tufts of fasciculate hyphae; hairs 200–1000 μm long, in fascicles of 12–20, partly branched, agglutinate, apically intertwined, brown, cylindrical hyphae with free apices. Secondary tomentum discernible only in microscopic sections; hairs 10–35 μm long, composed of single, branched, moniliform hyphae with free apices. Rhizines present but short, scattered towards the thallus centre. Cyphellae rather dense, (20–)60–100 per cm2, rounded, plane, erumpent to prominent, white, strongly contrasting with the dark tomentum; pore 0.3–1(–1.8) mm diam.; cells of basal membrane lacking papillae.
Apothecia not rare, often on thalli lacking phyllidia, mostly submarginal, dispersed, sessile to substipitate with a pronounced invagination on the underside, 2–4 mm diam., 0.5–0.6 mm high; disc orange-brown; proper margin verrucose to crenulate, sometimes thinly pilose when young, dark brown. Excipulum 100–150 μm broad. Hymenium 115–155 μm high; epithecium 2.5–5 μm thick, orange-brown. Ascospores fusiform, 1–3-septate, 27–38 × 5.5–7.5 μm.
Secondary chemistry
No substances detected by TLC; medulla and basal membrane of cyphellae K+ yellowish.
Distribution and ecology
Sticta andina is apparently native to the Neotropics and found most frequently in the northern Andes (Moncada et al. Reference Moncada, Mercado-Díaz, Smith, Bungartz, Sérusiaux, Lumbsch and Lücking2021). In Hawaii, this species is known from Hawaii, Kauai, Maui and Oahu, with many collections originating from introduced conifer forest on Maui. A lack of deviation in the ITS sequences compared to the main haplotype found in the northern Andes suggests the records from Hawaii to be the result of recent, perhaps anthropogenic long-distance dispersal (Moncada et al. Reference Moncada, Lücking and Lumbsch2020). In the northern Andes, Sticta andina is an upper montane to alpine taxon, typically confined to undisturbed forest and shrubby habitats, where it grows on trees and shrubs, rarely on rock. In Hawaii it was found in partially disturbed native rainforest and gulches of adjacent upland dry forest, between 1200 and 2400 m, with most collections originating from between 1500 and 2000 m. Reported phorophytes mostly include Cheirodendron and Metrosideros, but also Coprosoma (montana), Leptocophylla, Myrsine, Sophora (chrysophylla) and Vaccinium.
Remarks
Sticta andina was described recently to accommodate a common taxon mostly occurring in the northern Andes. It corresponds to the S. weigelii morphodeme but is not related to S. weigelii s. str., differing in the thick lower tomentum and whitish cyphellae lacking yellow pigment (Moncada et al. Reference Moncada, Mercado-Díaz, Smith, Bungartz, Sérusiaux, Lumbsch and Lücking2021). Sticta andina is characterized by a rather robust thallus with an olive-brown colour when fresh (yellowish brown in the herbarium), with predominantly marginal, rather dark, mostly flattened isidia and phyllidia. It can be distinguished from S. scabrosa subsp. hawaiiensis by the glabrous, more or less shiny lobe surface, by the isidia and phyllidia being darker than the thallus, and by the uniformly dark lower tomentum. Its ecology is also different, being found mostly in higher elevation rainforest, whereas S. scabrosa subsp. hawaiiensis is a weedy taxon typically occurring in lower altitude rainforest or secondary and anthropogenic vegetation. Sticta waikamoi is quite similar to S. andina and co-occurs with it in the same habitats, but is a smaller lichen overall, with narrower lobes forming cylindrical to somewhat flattened isidia and conspicuous rhizines; in addition, it often also grows on the ground.
Specimens examined
USA: Hawaii: Hawaii, Mauna Loa, Pohakuloa training area, 1585 m, 1977, P. K. Higashino 327 (HAW); Kauai, West Kauai, Koke'e State Park, Pihea Trail, 22°08ʹ51ʺN, 159°37ʹ53ʺW, 1250–1350 m, 2013, B. Moncada, R. Lücking & T. Flynn 7047 (B, PTBG); Maui, East Maui, Kipahulu Forest Reserve, 1385 m, 1976, P. K. Higashino & G. Mizuno 2034 (HAW); Maui, East Maui, Makawao Forest Reserve, 1800 m, 1975, C. W. Smith 1922 (HAW); Maui, East Maui, Haleakalā Volcano, 1890–1950 m, 1981, C. W. Smith 5074 (HAW); Maui, East Maui, Haleakalā Volcano, Haleakalā National Park, 2100 m, 1975, C. W. Smith 2105, 2164, 2168 (HAW); Maui, East Maui, Haleakalā Volcano, Haleakalā National Park, Halemauu Trail, 2400 m, 1975, C. W. Smith 2075 (HAW); Maui, East Maui, Haleakalā Volcano, Lower Waikamoi Preserve, 20°48ʹ23ʺN, 156°15ʹ19ʺW, 1200–1300 m, 2013, B. Moncada, R. Lücking & P. Bily 6951 (F); Maui, East Maui, Haleakalā Volcano, Upper Waikamoi Preserve, 20°46ʹ07ʺN, 156°14ʹ17ʺW, 1800–2100 m, 2013, B. Moncada, R. Lücking & P. Thomas 6983 (B), 6984 (F), 6997 (B, F); Maui, Olinda, Koolau Forest Reserve North Haleakala, 910 m, 1992, R. Rosentreter 8108 (SRP); Maui, Kipahulu Valley, Haleakalā National Park, 2100 m, 1984, A. C. Medeiros & D. Miranda 322 (HAW); Maui, Paliku, 2130 m, 1976, C. W. Smith 3091 (HAW); Oahu, Waianae Mountain Range, Mt Kaala, 1212 m, 1980, G. Y. Daida 628 (HAW); Oahu, Gully en route from Kuiki to Paliku, 2130 m, 1975, C. W. Smith 2220 (HAW).
Sticta antoniana Moncada & Lücking
Moncada et al., Pl. Fung. Syst. 65, 108 (2020); type: USA, Hawaii, Kauai, West Kauai, Koke'e State Park, Pihea Trail, 22°08ʹ51ʺN, 159°37′53″W, 1250–1350 m, 2013, B. Moncada, R. Lücking & T. Flynn 7053 (F—holotype!; B, HAW—isotypes!).
Primary photobiont cyanobacterial (Nostoc). Stipe absent. Thallus monophyllous, irregularly orbicular, to 10 cm across, frequently anisotomously branched; lobes to 7 mm broad, flabellate to truncate, ascending, not usually overlapping, their margins dissected into elongate lobules. Upper surface even, olive green when fresh, brownish grey when dry, glabrous, without papillae or pruina, with or without scattered, indistinct maculae. Marginal lobules abundant, much branched, 1–3(–5) mm long and 0.5–1 mm broad, concolorous with the thallus. Lower surface slightly uneven, whitish. Primary tomentum thick and dense, forming light grey-brown, arachnoid tufts of fasciculate hyphae except for a 2–5 mm broad marginal zone abruptly bare of tomentum; hairs 500–1500 μm long, formed of densely entangled, mostly unbranched, pale brown, cylindrical hyphae with free apices. Secondary tomentum inconspicuous; hairs scattered, 20–30 μm long, comprising single, unbranched, cylindrical, colourless hyphae. Cyphellae dense, 21–60 per cm2, rounded, plane, immersed, white; pore 0.1–0.3 mm diam.; cells of basal membrane irregularly bulging but lacking distinct papillae.
Apothecia common, laminal, sessile, 0.7–1.5 mm diam., initially zeorine with hairy margins but mature apothecia biatorine, with remnants of thallus layer basally and with scattered marginal hairs; disc dark reddish brown; margin crenulate, cream to pale orange. Ascospores oblong-fusiform, 3-septate, 40–50 × 6–7 μm.
Secondary chemistry
No substances detected by TLC; medulla K−, cyphellae K−.
Distribution and ecology
This species is thus far known only from the islands of Maui and Kauai where it is apparently restricted to more or less undisturbed or little disturbed montane rainforest at mid elevations (between 1200 and 1350 m). It grows on shaded bark of unidentified phorophyte trees.
Remarks
Sticta antoniana belongs to the S. tomentosa complex and cannot be phylogenetically separated from the latter on ITS sequences alone (Moncada et al. Reference Moncada, Lücking and Lumbsch2020). Due to its distinctive morphology, differing from typical S. tomentosa by the highly dissected lobe margins and the thick lower tomentum that leaves an abrupt bare margin, it is considered a separate species. Highly disparate, discrete morphologies in phylogenetically closely related lineages that may not be resolvable with standard barcoding markers are not rare in island biota, also being found in vascular plants (Baldwin et al. Reference Baldwin, Kyhos, Dvorak and Carr1991; Baldwin & Sandersson Reference Baldwin and Sanderson1998; Carlquist et al. Reference Carlquist, Baldwin and Carr2003). In lichens, similar phenomena were also demonstrated for Hawaiian Lobariella and Pseudocyphellaria (Moncada et al. Reference Moncada, Lücking and Suárez2014b; Lücking et al. Reference Lücking, Moncada and Smith2017a). The view that S. antoniana is a distinctive lineage not resolved by the ITS barcoding marker, and not a habitat-induced morph, is supported by the notion that the S. antoniana morphodeme is not known in populations of S. tomentosa outside Hawaii.
A recent example demonstrating a case where the ITS barcoding marker does not resolve two closely related species is the pair Neuropogon antarcticus (Du Rietz) I. M. Lamb versus Neuropogon aurantiacoater (Jacq.) I. M. Lamb, as shown by RADseq data (Grewe et al. Reference Grewe, Lagostina, Wu, Printzen and Lumbsch2018). The latter is a promising method to test cases where ITS exhibits limits of resolution, such as in S. antoniana versus S. tomentosa.
Additional specimens examined
USA: Hawaii: Maui, East Maui, Haleakalā Volcano, Lower Waikamoi Preserve, 20°48′23″N, 156°15′19″W, 1200–1300 m, 2013, B. Moncada, R. Lücking & P. Bily 6947 (B, F, HAW), 6948 (B, F); Kauai, West Kauai, Koke'e State Park, Pihea Trail, 22°08′51″N, 159°37′53″W, 1250–1350 m, 2013, B. Moncada, R. Lücking & T. Flynn 7045a (F), 7065 (F).
Sticta emmanueliana Moncada, Lücking & Lumbsch
Moncada et al., Pl. Fung. Syst. 65, 109 (2020); type: USA, Hawaii, Kauai, West Kauai, Koke'e State Park, Pihea Trail, 22°08′51″N, 159°37′53″W, 1250–1350 m, 2013, B. Moncada, R. Lücking & T. Flynn 7058 (F—holotype!; B, HAW—isotypes!).
Primary photobiont cyanobacterial (Nostoc). Stipe present, short and rather broad. Thallus mono- to polyphyllous, with individuals irregularly arranged, delicate, irregularly branched; lobes to 4 mm broad, irregularly elongate to tapering or truncate, horizontal to ascending, not usually overlapping, plane to slightly involute, their margins not thickened. Upper surface even, olive-brown to dark brown when fresh, brownish grey when dry, glabrous, without papillae or pruina, with scattered, white maculae; marginal cilia present, formed by submarginal tufts of primary tomentum that become visible beyond the margins, conspicuous particularly in younger lobes, evanescent in old lobes, brownish black, 0.1–0.4 mm long. Isidia present, predominantly marginal, 0.1–0.3 mm long and 0.05–0.1 mm broad, darker grey-brown, shiny, cylindrical, branched and becoming coralloid to arbuscular; arbusculae to 0.5 mm long and broad, their base lacking cyphellae. Lower surface uneven, mostly dark brown but marginally becoming white to cream. Primary tomentum becoming thin towards the margins, except for the cilia, otherwise forming loosely to densely arranged, brown tufts of fasciculate hyphae, much shorter and lighter than the cilia; hairs 100–200 μm long, in fascicles of 10–20, mostly unbranched but loosely agglutinate, cylindrical hyphae with free apices, usually brownish. Secondary tomentum developed to the margins, thin, pubescent, whitish to light brownish; hairs 25–100 μm long, comprising single, mostly unbranched, cylindrical hyphae with free apices, somewhat pale brown. Cyphellae dense, 41–80 per cm2, rounded, immersed-erumpent, white, appearing pruinose, with cream to light brown margins bare of tomentum; pore 0.1–0.2 mm diam. towards the margins, 0.2–0.5 mm diam. towards the centre; cells of basal membrane irregularly bulging but without distinct papillae.
Apothecia not observed.
Secondary chemistry
No substances detected by TLC; medulla K− to K+ pale yellow.
Distribution and ecology
This species was found on the islands of Maui and Kauai, at altitudes between 1200 and 1800 m, chiefly at the same localities as Sticta antoniana. Like that species, it also occurred in rather undisturbed montane rainforest at mid to higher elevations, growing on shaded tree bark including branches, for example on Myrsine, usually associated with bryophyte mats.
Remarks
On account of its marginal isidia and black cilia, Sticta emmanueliana comes morphologically close to Sticta cometiella Vain., described from Mexico. The latter appears to be strictly neotropical and is only very distantly related, clustering in a different clade of the global Sticta phylogeny (Moncada et al. Reference Moncada, Lücking and Lumbsch2020). It also frequently produces laminal isidia. The Australasian S. duplolimbata (Hue) Vain. is also similar (Galloway Reference Galloway1998, Reference Galloway and McCarthy2001) but likewise only distantly related (Moncada et al. Reference Moncada, Lücking and Lumbsch2020). Within Hawaiian Sticta, the species is best recognized by its dark marginal cilia.
Additional specimens examined
USA: Hawaii: Maui, East Maui, Haleakalā Volcano, Lower Waikamoi Preserve, 20°48′23″N, 156°15′19″W, 1200–1300 m, 2013, B. Moncada, R. Lücking & P. Bily 6949 (F), 6954 (B, F, HAW), 6955 (F); Maui, East Maui, Makawao Forest Reserve, 1800 m, 1975, C. W. Smith 1922 (HAW); Kauai, West Kauai, Koke'e State Park, Pihea Trail, 22°08′51″N, 159°37′53″W, 1250–1350 m, 2013, B. Moncada, R. Lücking & T. Flynn 7045a (F), 7064 (F).
Sticta flynnii Moncada & Lücking
Moncada et al., Pl. Fung. Syst. 65, 110 (2020); type: USA, Hawaii, Kauai, West Kauai, Koke'e State Park, Pihea Trail, 22°08′51″N, 159°37′53″W, 1250–1350 m, 2013, B. Moncada, R. Lücking & T. Flynn 7055 (F—holotype!).
Primary photobiont cyanobacterial (Nostoc). Stipe present, short and broad. Thallus mono- to polyphyllous, with one to few individuals that are irregularly arranged, delicate, unbranched to sparsely branched, becoming apically dissected; lobes to 7 mm broad, suborbicular to reniform or truncate, ascending, not usually overlapping, plane to involute, their margins becoming coarsely dissected. Upper surface even, olive green when fresh, bluish grey when dry, glabrous, with or without scattered papillae, without pruina, with scattered, white maculae. Isidia present, abundant, mostly marginal but also laminal, isidia 0.1–0.2 mm long and 0.05–0.1 mm broad, darker brown, shiny, cylindrical to somewhat flattened, arbuscular, branched and becoming coralloid; arbusculae to 2 mm long and broad, their base lacking cyphellae. Lower surface uneven, forming shallow, vein-like ridges, white to cream. Primary tomentum absent. Secondary tomentum developed to the margins, thin, pubescent, white; hairs 20–50 μm long, comprising single, mostly unbranched, cylindrical hyphae with free apices. Cyphellae scattered, 1–20 per cm2 towards the thallus centre and 21–40 per cm2 towards the margin, rounded to irregular or becoming angular, plane, immersed, white, appearing pruinose; pore 0.5–1 mm diam. towards the margins, 1–3 mm across towards the centre; cells of basal membrane irregularly bulging but without distinct papillae.
Apothecia not observed.
Secondary chemistry
No substances detected by TLC; medulla and membrane of cyphellae K−.
Distribution and ecology
This species has been found at the type locality on Kauai and one additional site on Maui. It was found in more or less undisturbed montane rainforest at mid to high elevations (between 1250 and 2300 m) on shaded tree bark associated with bryophytes. Given that it is a rather small species, it is likely to have been overlooked and might be more common.
Remarks
Together with Sticta acyphellata and S. smithii, S. flynnii is one of three Hawaiian species with small, caulescent, isidiate thalli. Sticta acyphellata is distinguished by the complete lack of cyphellae, whereas S. smithii differs in the small cyphellae and thicker tomentum. Several other, non-Hawaiian taxa share a caulescent, isidiate thallus, including S. brevipes (Müll. Arg.) Zahlbr., S. cyphellulata (Müll. Arg.) Hue, S. hypochra Vain., S. longipes (Müll. Arg.) Malme and S. marginifera Mont. (Galloway Reference Galloway1994a, Reference Galloway1998). These are all phylogenetically distinct (Moncada et al. Reference Moncada, Lücking and Lumbsch2020) and differ in their more robust thalli, as well as in other details. Notably, even the larger species do not appear to produce cyphellae as large as those found in S. flynnii.
Additional specimens examined
USA: Hawaii: Maui, East Maui, Haleakalā Volcano, Haleakalā National Park, 2233 m, 1975, C. W. Smith 1754 (HAW); Kauai, West Kauai, Koke'e State Park, Pihea Trail, 22°08′51″N, 159°37′53″W, 1250–1350 m, 2013, B. Moncada, R. Lücking & T. Flynn 7059 (HAW).
Sticta fuliginosa (Hoffm.) Ach.
Meth. Lichenum, 280 (1803).—Lobaria fuliginosa Hoffm., Deutschl. Flora 2, 109 (1796); type: United Kingdom, Wales, Cader Idris, Icon. in Dillenius, Historia Muscorum, tab. 26, fig. 100A (1742; lectotype fide Laundon (Reference Laundon1984), 218–219); corresponding specimen from hb. Dillenius (OXF—epitype fide Jørgensen & Tønsberg (Reference Jørgensen, Tønsberg, Ahti, Jørgensen, Kristinsson, Moberg, Søchting and Thor2007), 146).
Primary photobiont cyanobacterial (Nostoc). Stipe absent. Thallus mono- to polyphyllous; individuals robust, unbranched to sparsely branched; lobes 5–8(–12) cm broad, suborbicular, ascending, imbricate, plane to slightly revolute, margins entire to irregular. Upper surface uneven to shallowly scrobiculate, dark brown-grey when fresh, greyish brown when dry, glabrous, with or without scattered papillae, without pruina, with or without indistinct maculae. Isidia present, laminal, to 0.5 mm long and broad, dark grey to brownish grey, shiny, cylindrical to somewhat flattened, branched and becoming coralloid. Lower surface slightly uneven, white to cream. Primary tomentum rather thin and becoming thinner along the margins, whitish to pale brownish, forming somewhat scattered to more densely arranged tufts of fasciculate hyphae; hairs 100–200 μm long, in fascicles of 10–20, mostly unbranched but agglutinate, cylindrical hyphae with free apices. Secondary tomentum not discernible. Cyphellae numerous, 20–60 per cm2, rounded to usually angular, plane, immersed, white to cream; pore 0.5–3(–5) mm diam.; cells of basal membrane without papillae.
Apothecia not observed.
Secondary chemistry
No substances detected by TLC; medulla K−, membrane of cyphellae K−.
Distribution and ecology
Sticta fuliginosa in the strict sense is a subcosmopolitan species, with specimens confirmed through sequence data known from most regions of the world, including Hawaii (Magain & Sérusiaux Reference Magain and Sérusiaux2015; Moncada et al. Reference Moncada, Lücking and Lumbsch2020). Although exhibiting little genetic variation, there is some subtle geographical structure in the ITS sequence data, suggesting a very recent, rapid, yet natural expansion of this taxon. In Hawaii, it is usually epiphytic in more or less undisturbed rainforest habitats and gulches in adjacent dry forest. It tolerates a certain degree of disturbance and may also be found in secondary or anthropogenic vegetation, as records from introduced trees in botanical gardens demonstrate. Together with S. tomentosa, S. fuliginosa is the most common and widespread Sticta species in Hawaii, with a well-documented, broad altitudinal range between under 500 to over 2300 m. Identified phorophytes include indigenous species of Cordyline (fruticosa), Dodonaea (viscosa), Leptecophylla, Metrosideros (collina), Rubus (hawaiiensis), and Sophora (chrysophylla).
Remarks
The name Sticta fuliginosa has traditionally been applied to all forms with more or less broad, rounded lobes with laminal isidia (Joshi & Awasthi Reference Joshi and Awasthi1982; Swinscow & Krog Reference Swinscow and Krog1988; Galloway Reference Galloway1994a, Reference Galloway and McCarthy2001, Reference Galloway2007; Galloway et al. Reference Galloway, Stenroos and Ferraro1995; Büdel et al. Reference Büdel, Meyer, Salazar, Zellner, Zotz and Lange2000; Brodo et al. Reference Brodo, Sharnoff and Sharnoff2001; Farkas Reference Farkas2003; Galloway & Thomas Reference Galloway, Thomas, Nash, Ryan, Diederich, Gries and Bungartz2004; Jørgensen & Tønsberg Reference Jørgensen, Tønsberg, Ahti, Jørgensen, Kristinsson, Moberg, Søchting and Thor2007; Makryi Reference Makryi2008; Smith et al. Reference Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009). Molecular phylogenetic data, however, have demonstrated that this morphodeme corresponds to more than a dozen, mostly unrelated species (Moncada et al. Reference Moncada, Reidy and Lücking2014a, Reference Moncada, Suárez and Lücking2015, Reference Moncada, Lücking and Lumbsch2020; Magain & Sérusiaux Reference Magain and Sérusiaux2015). Surprisingly, even in a narrow sense supported by molecular data, S. fuliginosa s. str. remains the most widespread species of the genus. Morphologically, it is best characterized (and separated from similar species) by the rather robust thalli, the glabrous lobe surface, the cylindrical isidia, the whitish underside with rather large, plane cyphellae that often become angular in outline, and the lack of papillae on the cells of the membrane of the cyphellae. The only similar species in Hawaii is S. hawaiiensis, which differs in the much smaller thalli and lobes, the distinct, white, marginal cilia, and the numerous papillae on the membrane of the cyphellae.
Specimens examined
USA: Hawaii: Hawaii, Mauna Loa, Pohakuloa training area, 1585 m, 1977, P. K. Higashino 525 (HAW); Hawaii, Puu Huluhulu Saddle Road, 19°51′20″N, 155°08′56″W, 2365 m, 1992, W. L. Culberson 22164 (DUKE); Kauai, Koke'e State Park, Kalua Puhi Trail, 1070 m, 1985, W. A. Weber & D. Randolph L-77946 (US); Kauai, West Kauai, Koke'e State Park, Mohihi Trail, 467 m, 1979, G. Y. Daida 267 (HAW); Kauai, West Kauai, Koke'e State Park, Pihea Trail, 22°08′51″N, 159°37′53″W, 1250–1350 m, 2013, B. Moncada, R. Lücking & T. Flynn 7043 (B), 7052 (F); Kauai, West Kauai, Waimea Canyon, Iliau Nature Loop & Kukui Trail at Kokee Road, 22°03′04″N, 159°39′36″W, 900 m, 2013, B. Moncada, R. Lücking & T. Flynn 7023 (B, F), 7026 (B, F, HAW); Kauai, Alakai Swamp, C. W. Smith 6179 (HAW); Maui, East Maui, Haleakalā National Park, 2070 m, 1977, C. W. Smith 3343 (HAW); Maui, Auahi Forest Reserve, 760 m, 1992, P. DePriest 8213 (US); Maui, East Maui, Haleakalā Volcano, 1890–1950 m, 1981, C. W. Smith 5072 (HAW); Maui, East Maui, Haleakalā Volcano, Haleakalā National Park, 2233 m, 1975, C. W. Smith 1762 (HAW); Maui, East Maui, Haleakalā Volcano, Lower Waikamoi Preserve, 20°48′23″N, 156° 15′19″W, 1200–1300 m, 2013, B. Moncada, R. Lücking & P. Bily 6945 (F); Maui, East Maui, Haleakalā Volcano, Upper Waikamoi Preserve, 20°46′07″N, 156°14′17″W, 1800–2100 m, 2013, B. Moncada, R. Lücking & P. Thomas 6978 (B, F, HAW), 6979 (F), 6982 (HAW), 6988 (B, F), 6990 (F), 6994 (F), 6998 (HAW); Maui, East Maui, Kipahulu Forest Reserve, 1160 m, 1976, P. K. Higashino & G. Mizuno 2551 (HAW); Maui, Olinda, Koolau Forest Reserve, 20°49′23″N, 156°16′12″W, 910 m, 1992, R. Rosentreter 8085, 8087, 8101 (SRP); Oahu, Honolulu, Ho'omaluhia Botanical Garden, 21°23′08″N, 157°48′16″W, 1991, S. C. Tucker 30518 (LSU); Oahu, Honolulu, Wahiawa Botanical Garden, 21°25′N, 158°00′W, 1991, S. C. Tucker 30485 (LSU); Oahu, Waahila Ridge, 275 m, 1979, G. Y. Daida 251 (HAW); Oahu, Waianae Mountains, Honouliuli Forest Reserve, 945 m, 1978, C. W. Smith 4112 (HAW); Oahu, Waianae Mountains, Kaua Trail, 945 m, 1978, G. Y. Daida 203 (HAW).
Sticta hawaiiensis Moncada & Lücking
Moncada et al., Pl. Fung. Syst. 65, 111 (2020); type: USA, Hawaii, Kauai, West Kauai, Koke'e State Park, Pihea Trail, 22°08′51″N, 159°37′53″W, 1250–1350 m, 2013, B. Moncada, R. Lücking & T. Flynn 7048 (F—holotype!; HAW—isotype!).
Primary photobiont cyanobacterial (Nostoc). Stipe indistinct or absent. Thallus mono- to polyphyllous, with one to few individuals that are irregularly arranged, delicate, unbranched; lobes 0.5–1 cm broad, suborbicular, ascending, not usually overlapping, plane to revolute, margins entire to irregular. Upper surface irregularly verruculose-rugose, dark grey when fresh, dark bluish grey when dry, thinly whitish tomentose, with or without scattered papillae, without pruina, without distinct maculae; marginal cilia abundant and distinct, particularly in younger thalli, white, 0.2–0.7 mm long; hairs of upper tomentum 10–20 μm long, of single, branched, moniliform hyphae with free apices. Isidia present, laminal, to 0.2 mm long and broad, dark grey to brownish grey, shiny, cylindrical, branched and becoming somewhat coralloid. Lower surface slightly uneven, white to cream. Primary tomentum developed except along the margins, forming somewhat scattered to more densely arranged, mottled brown tufts of fasciculate hyphae; hairs 100–150 μm long, in fascicles of 10–20, mostly unbranched but agglutinate, cylindrical hyphae with free apices. Secondary tomentum developed to the margins, thin, pubescent, white; hairs 10–15 μm long, comprising single, branched, strongly moniliform hyphae with globose cells and free apices. Cyphellae scattered, 1–20 per cm2, rounded, plane, immersed to becoming erumpent, white; pore 0.1–0.2 mm diam.; cells of basal membrane irregularly bulging, with numerous tiny papillae per cell.
Apothecia not observed.
Secondary chemistry
No substances detected by TLC; medulla K−, membrane of cyphellae K−.
Distribution and ecology
The ecology of this putatively endemic species is rather similar to that of Sticta antoniana and S. emmanueliana, as it was found chiefly at the same localities on the islands of Maui and Kauai, between 1200 and 1300 m. It occurs in rather undisturbed montane forest at mid elevations, on shaded tree bark over and between bryophyte mats. However, an older collection was identified from Oahu, in non-indigenous rainforest at lower altitude.
Remarks
This species belongs to the apparently pantropical Sticta ciliata complex which appears to contain several recently-diverged, phenotypically cryptic lineages (Magain & Sérusiaux Reference Magain and Sérusiaux2015; Mercado-Díaz et al. Reference Mercado-Díaz, Lücking, Moncada, Widhelm and Lumbsch2020; Moncada et al. Reference Moncada, Lücking and Lumbsch2020). Species of this clade resemble S. fuliginosa with respect to their rounded lobes with laminal isidia but are much smaller overall and are consistently set apart by the conspicuous, white marginal cilia.
Additional specimens examined
USA: Hawaii: Oahu, Koolau Mountains, Tantalus, 467 m, 1977, G. Y. Daida 520 (HAW); Maui, East Maui, Haleakalā Volcano, Lower Waikamoi Preserve, 20°48′23″N, 156°15′19″W, 1200–1300 m, 2013, B. Moncada, R. Lücking & P. Bily 6953 (B, F, HAW).
Sticta limbata (Sm.) Ach.
Meth. Lichenum, 280 (1803).—Lichen limbatus Sm. in Smith & Sowerby, Engl. Bot., 16, tab. 1104 (1803); type: United Kingdom, Wales, Cardiganshire, Haford, J. E. Smith s. n. (BM-BM000763531—lectotype!).
Primary photobiont cyanobacterial (Nostoc). Stipe absent. Thallus mono- to more rarely polyphyllous; individuals small, unbranched to sparsely branched; lobes 1–3 cm broad, suborbicular, ascending, imbricate, plane, their margins entire to irregular. Upper surface uneven, brown-grey when fresh, greyish brown when dry, glabrous, with or without scattered papillae, without pruina, with or without indistinct maculae. Soredia present, marginal, forming labriform soralia, white-grey. Lower surface slightly uneven, white to cream. Primary tomentum rather thin and becoming thinner along the margins, whitish to pale brownish, forming somewhat scattered to more densely arranged tufts of fasciculate hyphae; hairs 100–150 μm long, in fascicles of 10–20, mostly unbranched, agglutinate, cylindrical hyphae with free apices. Secondary tomentum not discernible. Cyphellae numerous, 20–60 per cm2, rounded to usually angular, plane, immersed, white to cream; pore 0.5–3 mm diam.; cells of basal membrane without papillae.
Apothecia not observed.
Secondary chemistry
No substances detected by TLC; medulla K−, membrane of cyphellae K−.
Distribution and ecology
Similar to Sticta fuliginosa, S. limbata is a subcosmopolitan species with specimens confirmed through sequence data, known from many regions of the world including Hawaii (Moncada et al. Reference Moncada, Lücking and Lumbsch2020). Sticta limbata usually grows epiphytically on trunks and branches in undisturbed habitats. It is generally much rarer than S. fuliginosa, although the known collections indicate a similarly broad altitudinal range between 900 and 2100 m.
Remarks
The formation of soredia is remarkably rare in the genus Sticta. Even so, sorediate forms traditionally identified with the name S. limbata represent several unrelated species (Moncada et al. Reference Moncada, Reidy and Lücking2014a). Sticta limbata s. str. is characterized by suborbicular lobes with a whitish underside and cyphellae that become rather large and angular, similar to those of the closely related S. fuliginosa. In Hawaii, the species is unmistakable.
Specimens examined
USA: Hawaii: Kauai, West Kauai, Waimea Canyon, Iliau Nature Loop & Kukui Trail at Kokee Road, 22°03′04″N, 159°39′36″W, 900 m, 2013, B. Moncada, R. Lücking & T. Flynn 7024 (B, F); Maui, East Maui, Haleakalā Volcano, Upper Waikamoi Preserve, 20°46′07″N, 156°14′17″W, 1800–2100 m, 2013, B. Moncada, R. Lücking & P. Thomas 6995 (HAW), 6999 (F).
Sticta plumbicolor (Zahlbr.) Zahlbr.
Cat. Lich. Univers. 3, 398 (1925).—Stictina plumbicolor Zahlbr., Annls Mycol. 1, 356 (1903); type: USA, Hawaii, Molokai, 760 m, on shrubs, D. D. Baldwin 17 (W—holotype!).
Primary photobiont cyanobacterial (Nostoc). Stipe absent or indistinct (in young thalli). Thallus monophyllous, irregularly orbicular to palmate, to 5 cm across, anisotomously to polytomously branched; lobes 3–6 mm broad, flabellate to truncate, horizontal, not usually overlapping, their margins entire to slightly crenate. Upper surface even, bluish grey when fresh, bluish to yellowish grey when dry, glabrous, without papillae or pruina, with scattered, whitish maculae, with marginal, fasciculate, white cilia. Phyllidia present, predominantly marginal but frequently also extending onto the lamina, to 0.7 mm long and broad, dark bluish grey, darker than the thallus, branched and forming coralloid to palmate clusters with a basal, flattened stipe lacking cyphellae. Lower surface uneven to ridged-scrobiculate, white to pale cream (becoming yellow-orange in the herbarium). Primary tomentum thin, sparse towards the margins, fasciculate, whitish to pale yellowish grey; hairs 70–250 μm long, in fascicles of 10–20, mostly unbranched but agglutinate, colourless, cylindrical hyphae with free apices. Secondary tomentum thinly arachnoid, whitish; hairs scattered, 10–20 μm long, comprising single, unbranched, moniliform, colourless hyphae. Cyphellae scattered, 20–40(–60) per cm2, rounded to somewhat irregular in outline, plane, immersed-erumpent, white; pore 0.1–0.3 mm diam.; cells of basal membrane with 2–4 papillae per cell.
Apothecia present, submarginal, sessile, 0.8–1.5 mm diam., biatorine, glabrous or rarely with scattered marginal hairs; disc reddish brown; margin smooth to minutely crenulate, cream to pale orange. Ascospores oblong-fusiform, (1–)3-septate, 30–40 × 7–8 μm.
Secondary chemistry
No substances detected by TLC; medulla K−, cyphellae K−.
Distribution and ecology
Sticta plumbicolor is known with certainty only from Hawaii. Reports from other regions, such as Jamaica (Minter et al. Reference Minter, Rodriguez-Hernández and Mena-Portales2001; three collections housed at DUKE: 1967, C. Racine s. n.; 1968, W. L. Culberson & C. F. Culberson 13860, 13884), are misidentifications probably pertaining to S. filicinella (Nyl.) Zahlbr. In Hawaii, the species is found on all major islands (Kauai, Oahu, Molokai, Maui, Hawaii), generally in more or less undisturbed rainforests at low to mid elevation (between 75 and 640 m; a single outlier is from 2100 m). Notably, the closely related S. tomentosa, which cannot be separated using the ITS barcoding marker, is mostly found between 1000 and 2200 m (see below). Sticta plumbicolor grows mainly on tree trunks and branches, rarely on other substrata, often associated with bryophytes; identified phorophytes include Cordyline (fruticosa), Metrosideros, Myrsine and Psidium (cattleianum).
Remarks
Prior to our molecular phylogenetic revision of Hawaiian Sticta (Moncada et al. Reference Moncada, Lücking and Lumbsch2020), S. plumbicolor was considered to be the only potentially endemic taxon, bearing in mind that reports from Jamaica were erroneous (see above). To our surprise, S. plumbicolor proved to be phylogenetically indistinguishable from S. tomentosa based on the ITS barcoding marker. However, for the time being it is retained as a separate taxon, given the discrete morphological differences, providing a similar case to that of S. antoniana (see discussion above). Sticta plumbicolor is smaller and more delicate than S. tomentosa and forms abundant marginal and partly laminal clusters of conspicuous phyllidia. This morphology is not known from any collection outside Hawaii, while S. tomentosa in its typical form is a pantropical taxon. The most similar species appears to be the neotropical S. filicinella which produces delicate, cylindrical isidia instead of robust phyllidia.
Specimens examined
USA: Hawaii: Hawaii, east coast, Honolii area, 1977, P. K. Higashino & J. Green 324 (HAW); Maui, East Maui, Haleakalā National Park, Paliku cabin, 2100 m, 1975, C. W. Smith 2131 (HAW); Molokai, Wailau Valley, 150 m, 1977, L. Stemmermann 2062 (HAW); Oahu, Ewa District, Ewa Forest Reserve, 1975, D. Vitt 14519 (MIN); Oahu, Honolulu, Manoa Valley, Manoa Falls Trail, 75 m, 1992, B. D. Ryan 29039 (ASU); Oahu, Koolau Mountains, ridge from Tantalus to Puu Konahuanui, 1970, C. W. Smith 130c (HAW); Oahu, Koolau Mountains, Tantalus, Manoa Cliffs Trail, 467 m, 1977, G. Y. Daida 463 (HAW); Oahu, Koolau Range, Manoa Valley, Manoa Cliffs, 21°19′55″N, 157°48′43″W, 410–575 m, 2013, B. Moncada, R. Lücking & C. W. Smith 6910 (B, F, HAW), 6918a (F), 6925 (B); Oahu, Waianae Mountains, Makaleha Valley, Mokuleia Forest Reserve, 400 m, 1975, C. W. Smith 1717 (HAW).
Sticta scabrosa subsp. hawaiiensis Moncada, Lücking & C.W. Sm.
Moncada et al., Willdenowia (in press); type: USA, Hawaii, Oahu, Koolau Range, Manoa Valley, Manoa Cliffs Trail, 21°19′55″N, 157°48′43″W, 410–575 m, 2013, B. Moncada et al. 6915 (BISH—holotype!; B, F—isotypes!).
Primary photobiont cyanobacterial (Nostoc). Stipe absent. Thallus monophyllous, forming suborbicular rosettes or becoming irregular, to 20 cm across, anisotomously to polytomously branched; lobes 5–10 mm broad, flabellate, more or less horizontal, imbricate, undulate to slightly canaliculate, their margins entire to shallowly crenate. Upper surface uneven to foveolate-pitted, olive when fresh, greyish brown when dry, glabrous, with or without scattered papillae bearing tiny trichomes, without pruina but especially towards the margins thinly scabrous, with indistinct, cream maculae; rarely with marginal, pale to golden brown cilia. Phyllidia present, predominantly marginal but frequently also extending onto the lamina, to 0.5 mm long and broad, grey-brown, branched and becoming coralloid to palmate with a basal, flattened stipe lacking cyphellae. Lower surface uneven to undulate, dark brown to blackish. Primary tomentum dense but becoming sparse towards the lobe margins, spongy, forming pale to dark grey-brown, arachnoid tufts of fasciculate hyphae; hairs 200–1000 μm long, in fascicles of 6–12, partly branched, agglutinate, apically intertwined, pale to light brown, cylindrical hyphae with free apices. Secondary tomentum appressed, arachnoid; hairs 15–25 μm long, composed of single, branched, moniliform hyphae with free apices. Rhizines sparse. Cyphellae rather dense, 20–60 per cm2, rounded, plane, erumpent to prominent, cream; pore 0.5–1.2(–1.8) mm diam.; cells of basal membrane lacking papillae.
Apothecia not observed.
Secondary chemistry
No substances detected by TLC; medulla and basal membrane of cyphellae K+ (pale) ochraceous(-yellow).
Distribution and ecology
The typical subspecies, Sticta scabrosa Moncada et al. subsp. scabrosa, is widely distributed in the Neotropics, whereas subsp. hawaiiensis is known only from Hawaii (Moncada et al. Reference Moncada, Lücking and Lumbsch2020, Reference Moncada, Mercado-Díaz, Smith, Bungartz, Sérusiaux, Lumbsch and Lücking2021). Both subspecies share the same ecology, being found in more tropical climates than most other Sticta species and often in somewhat exposed microsites in disturbed or secondary and anthropogenic habitats, sometimes with a weedy character. The taxon typically grows on tree trunks but is also found occasionally on rocks and other substrata. Most specimens were collected below 1000 m altitude, more rarely up to 1350 m and exceptionally (two collections) between 1800 and 2130 m. The broad range of reported phorophytes includes various native and introduced taxa: Araucaria, Casuarina, Cocos (nucifera), Cordyline (fruticosa), Eucalyptus, Eugenia (cumini), Leptecophylla (tameiameiae), Litchi (chinensis), Metrosideros (collina, tremuloides), Myrsine, Osteomeles (anthyllidifolia), Pisonia, Sapindus, Schinus (terebinthifolius), Stachytarpheta (jamaicensis) and Trema (micrantha).
Remarks
Sticta scabrosa is another recently established species of the S. weigelii morphodeme (Moncada et al. Reference Moncada, Mercado-Díaz, Smith, Bungartz, Sérusiaux, Lumbsch and Lücking2021). It is only distantly related to S. weigelii and differs in several important features: the opaque, apically thinly scabrous lobe surface, the flattened phyllidia that are concolorous with the thallus, the rather thick, paler lower tomentum, and the lack of a yellow pigment in the cyphellae. The Hawaiian material differs consistently from neotropical populations in two substitutions in the ITS region and was formally recognized as a subspecies (Moncada et al. Reference Moncada, Mercado-Díaz, Smith, Bungartz, Sérusiaux, Lumbsch and Lücking2021). The only similar taxa in Hawaii are S. andina and S. waikamoi, from which S. scabrosa subsp. hawaiiensis can be distinguished by the paler lower tomentum, the phyllidia being frequently laminal and concolorous with the thallus, the opaque lobe surface that becomes thinly scabrous towards the apices, and the sometimes scrobiculate-foveolate lobe tips. It also differs ecologically in its weedy character, being found mostly in somewhat exposed situations at lower altitudes. Specimens previously identified under the name S. beauvoisii (Elix & McCarthy Reference Elix and McCarthy1998, Reference Elix and McCarthy2008; Benner & Vitousek Reference Benner and Vitousek2012; Smith Reference Smith2013) largely correspond to this taxon.
Additional specimens examined
USA: Hawaii: Hawaii, Black Sand Beach, 1980, G. Y. Daida 594 (HAW); Hawaii, Hilo, Rainbow Falls, 70 m, 1976, C. W. Smith 2460 (HAW); Hawaii, North Kohala District, Hawi, 305 m, 1978, C. W. Smith 4366 (HAW); Kauai, South Kauai, Hawaii Agro Forestry Products Agro Forestry Demonstration plot, 21°57′49″N, 159°24′56″W, 140 m, 2013, B. Moncada, R. Lücking & T. Flynn 7076 (F, PTBG), 7078 (F); Kauai, South Kauai, Kahili Adventist School near Kahili Mountain Park, 21°57′49″N, 159°29′03″W, 275 m, 2013, B. Moncada, R. Lücking & T. Flynn 7069 (B, PTBG), 7070 (B, F), 7071 (PTBG), 7075 (B); Kauai, South Kauai, Kahili Ridge Trail, 21°57′52″N, 159°29′29″W, 300–450 m, 2013, B. Moncada, R. Lücking & T. Flynn 7072 (F), 7074 (F); Kauai, West Kauai, Koke'e State Park, Pihea Trail, 22°08′51″N, 159°37′53″W, 1250–1350 m, 2013, B. Moncada, R. Lücking & T. Flynn 7051 (F, HAW), 7054 (B, F, PTBG), 7056 (PTBG), 7057 (F), 7060 (B), 7063 (F); Kauai, West Kauai, Waimea Canyon, Iliau Nature Loop & Kukui Trail at Kokee Road, 22°03′04″N, 159°39′36″W, 900 m, 2013, B. Moncada, R. Lücking & T. Flynn 7025 (B, F, PTBG); Kauai, Koke'e State Park, Mohihi Trail, 1220 m, 1979, G. Y. Daida 287 (HAW); Kauai, Lawai Cemetery, 1978, G. Y. Daida 126 (HAW); Kauai, Na Pali, Kona Forest Reserve, 1035 m, 1975, P. K. Higashino 1018 (HAW); Kauai, Wahiawa Bog, 707 m, 1979, G. Y. Daida 293, 295 (HAW); Maui, East Maui, Makawao Forest Reserve, 1800 m, 1975, C. W. Smith 1924 (HAW); Maui, East Maui, Haleakalā Volcano, Haleakalā National Park, 2130 m, 1977, C. W. Smith 3256 (HAW); Maui, East Maui, Manawainui Valley, 1976, P. K. Higashino & G. Mizuno 2304 (HAW); Maui, East Maui, East Maui Irrigation (EMI), 20°53′06″N, 156°12′29″W, 225 m, 2013, B. Moncada & R. Lücking 6934 (B, F, HAW), 6935 (B, F), 6938 (F, HAW); Maui, East Maui, Hana Highway, Nua‘ailua Bay scenic lookout, 20°51′33″N, 156°09′13″W, 100 m, 2013, B. Moncada & R. Lücking 6937 (B, F, HAW); Maui, East Maui, Kipahulu Forest Reserve, 1160 m, 1976, P. K. Higashino & G. Mizuno 2588 (HAW); Maui, Kipahulu Valley, 520 m, 1980, G. Y. Daida 759 (HAW); Maui, West Maui Mountains, Maunalei Arboretum, 20°58′46″N, 156°37′14″W, 400 m, 2013, B. Moncada, R. Lücking & P. Thomas 7013 (F), 7014 (F), 7015 (B, F, HAW), 7018 (F), 7019 (HAW), 7020 (B, F, HAW); Molokai, Pakuwoaku Ridge, 150 m, 1977, L. Stemmermann 2048 (HAW); Molokai, Wailau Valley, 1975, P. K. Higashino 315 (HAW); Molokai, Kalaupapa Cliff Trail, 21°10′40″N, 157°00′13″W, 30–470 m, 1993, C. W. Smith s. n. (HAW); Oahu, Koolau Mountains, Kahuku Forest Reserve, 275 m, 1975, C. W. Smith 1648 (HAW); Oahu, Koolau Mountains, Likeke Trail, 300 m, 1975, C. W. Smith 1448 (HAW); Oahu, Koolau Mountains, Manoa Valley, Manoa Falls Trail, 1975, P. K. Higashino 160 (HAW); Oahu, Koolau Range, Manoa Valley, Manoa Cliffs Trail, 21°19′55″N, 157°48′43″W, 410–575 m, 2013, B. Moncada, R. Lücking & C. W. Smith 6911 (F, HAW), 6912 (F), 6914 (F), 6917 (F, HAW), 6919 (B, F, HAW), 6922 (B, F, HAW), 6924 (B, F), 6919b (B, F); Oahu, Niu Ridge, 290 m, 1980, G. Y. Daida 428 (HAW); Oahu, Upper Manoa Valley, 1978, S. Conant 304 (HAW); Oahu, Waahila Ridge, 275 m, 1979, G. Y. Daida 250 (HAW); Oahu, Waialae Iki Ridge, 1983, W. Char 312 (HAW); Oahu, Waianae Mountains, Honouliuli Forest Reserve, 945 m, 1978, C. W. Smith 4129 (HAW); Oahu, Waianae Mountains, Makua Keaau Forest Reserve, 250 m, 1975, E. Funk 302 (HAW); Oahu, Waianae Mountains, Mokuleia Forest Reserve, 550–670 m, 1977, L. Stemmermann 319 (HAW); Oahu, Waianae Range, Land of 10 000 Snails, 730 m, 2011, C. W. Smith s. n. (HAW); Oahu, Waianae Range, Makahela Valley, 1975, L. Yoshida 318 (HAW); Oahu, Kahuku, 275 m, 1979, E. Funk 311 (HAW); Oahu, summit of Waikane Trail, 395 m, 1977, G. Y. Daida 115 (HAW); Oahu, Tantalus, 460 m, 1979, K. Clark 4 (HAW); Oahu, Waahila Ridge Trail, 1979, C. S. Futa 47 (HAW).
Sticta smithii Moncada & Lücking
Moncada et al., Pl. Fung. Syst. 65, 113 (2020); type: USA, Hawaii, Oahu, Koolau Range, Manoa Valley, Manoa Cliffs Trail, 21°19′55″N, 157°48′43″W, 410–575 m, 2013, B. Moncada, R. Lücking & C. W. Smith 6916 (F—holotype!; B, HAW—isotypes!).
Primary photobiont cyanobacterial (Nostoc). Stipe present, short. Thallus mono- to polyphyllous, with one to few individuals that are irregularly arranged, delicate, irregularly branched and dissected; lobes to 0.5 mm broad, spathuliform to truncate, ascending, not usually overlapping, plane to involute, their margins becoming strongly dissected. Upper surface even, olive-grey when fresh, bluish grey when dry, glabrous, without papillae or pruina, with scattered, white maculae. Isidia present, predominantly marginal, 0.1–0.2 mm long and 0.05–0.1 mm broad, darker grey-brown, shiny, somewhat flattened, arbuscular, much branched and becoming coralloid; arbusculae to 1 mm long and broad, distinctly flattened, base lacking cyphellae. Lower surface uneven, forming shallow, vein-like ridges, white to cream, with pale orange streaks towards the centre. Primary tomentum dense but short, becoming even shorter towards the margins, whitish; hairs 50–100 μm long, of single to somewhat agglutinate, mostly unbranched, cylindrical, colourless hyphae with free apices. Secondary tomentum absent. Cyphellae rather dense, 21–40 per cm2, rounded to somewhat irregular, plane, immersed, white; pore 0.2–0.5 mm diam.; cells of basal membrane irregularly bulging, each cell with 1–3 elongated papillae, therefore appearing thorny.
Apothecia not observed.
Secondary chemistry
No substances detected by TLC; medulla K−, membrane of cyphellae K−.
Distribution and ecology
Sticta smithii is known from a partially disturbed secondary rainforest on the densely populated and strongly altered island of Oahu, growing in shaded conditions on tree trunks between bryophytes, at c. 500 m altitude. It was previously also collected on Maui, on Haleakalā Volcano, at an altitude of nearly 2000 m. This suggests that this small species has been overlooked and might be more common.
Remarks
Sticta smithii is similar to S. flynnii in that both have small, caulescent, isidiate thalli. The main difference between these two species is the size of the cyphellae: they remain small and rounded in S. smithii but become rather large and irregular in S. flynnii.
Additional specimen examined
USA: Hawaii: Maui, East Maui, Haleakalā Volcano, 1890–1950 m, 1981, C. W. Smith 5073 (HAW).
Sticta tomentosa (Sw.) Ach.
Meth. Lichenum, 279 (1803).—Lichen tomentosus Sw., Prodr., 147 (1788).—Lobaria tomentosa (Sw.) Räuschel, Nomenclat. Bot. 3, 330 (1797).—Stictina tomentosa (Sw.) Nyl., Syn. Meth Lich. 1(2), 343 (1860).—Dystictina tomentosa (Sw.) Clem., Gen. Fung., 175 (1909); type: Jamaica, unknown locality, Swartz s. n. (STB sheet 44, lectotype! fide Galloway (Reference Galloway1994b), 47).
Primary photobiont cyanobacterial (Nostoc). Stipe absent. Thallus monophyllous, irregularly orbicular to palmate, to 5 cm across, anisotomously to polytomously branched; lobes 7–15 mm broad, flabellate to truncate, horizontal to ascending, not usually overlapping, their margins entire to slightly crenate. Upper surface even, bluish grey when fresh, bluish to yellowish grey when dry, glabrous, without papillae or pruina, with scattered, whitish maculae, with marginal, fasciculate, white cilia. Isidia and soralia not observed. Lower surface uneven to ridged-scrobiculate, white to pale cream. Primary tomentum thin, sparse towards the margins, fasciculate to somewhat spongy towards the centre, whitish to pale grey; hairs 70–300(–500) μm long, in fascicles of 10–20, mostly unbranched but agglutinate, colourless, cylindrical hyphae with free apices. Secondary tomentum thinly arachnoid, whitish; hairs scattered, 10–25 μm long, composed of single, unbranched, moniliform, colourless hyphae. Cyphellae dense, (20–)40–60(–100) per cm2, rounded, plane, immersed-erumpent, white; pore 0.1–0.3(–0.5) mm diam.; cells of basal membrane with 2–4 papillae per cell.
Apothecia common, submarginal, sessile, 1–2 mm diam., biatorine, glabrous or with scattered marginal hairs; disc reddish brown; margin crenulate, cream. Ascospores oblong-fusiform, 1–3-septate, 30–45 × 6–10 μm.
Secondary chemistry
No substances detected by TLC; medulla K−, cyphellae K−.
Distribution and ecology
On the basis of molecular data, Sticta tomentosa is demonstrably a pantropical species (Moncada et al. Reference Moncada, Lücking and Lumbsch2020). It is found in more or less undisturbed, mid- to high-elevation rainforest habitats. In Hawaii it has been collected in numerous localities on all major islands, at altitudes between 500 and 2200 m but mostly above 1000 m. It typically grows on bark and is often associated with bryophytes, with the following identified phorophyte species: Broussasia (arguta), Dodonaea, Geranium (multiflorum), Metrosideros (collina, tremuloides), Myrsine and Pinus.
Remarks
Sticta tomentosa is characterized by the typically bluish grey thallus with ciliate margins, the numerous apothecia, and the white underside with a very thin tomentum and tiny, often barely discernible cyphellae (Galloway Reference Galloway1994b; Galloway & Thomas Reference Galloway, Thomas, Nash, Ryan, Diederich, Gries and Bungartz2004; Moncada Reference Moncada2012). In Hawaii, it is the only species that reproduces via apothecia and ascospores only and that does not form vegetative propagules or marginal lobules. Most similar is S. antoniana, but in that taxon, the lobe margins are deeply dissected into narrow lobules and the underside features a thick, grey-brown tomentum abruptly ending short of the margins and leaving the margins entirely bare.
Specimens examined
USA: Hawaii: Hawaii, Olaa Forest Tract, 1150 m, 1979, P. J. Burton 428 (HAW); Kauai, West Kauai, Koke'e State Park, Pihea Trail, 22°08′51″N, 159°37′53″W, 1250–1350 m, 2013, B. Moncada, R. Lücking & T. Flynn 7044 (F), 7050 (HAW), 7061 (B), 7062 (F), 7066 (F); Kauai, Kalalau Lookout area, 1220 m, 1979, G. Y. Daida 297, 304 (HAW); Kauai, Wahiawa Bog, 707 m, 1979, G. Y. Daida 293 (HAW); Maui, East Maui, Haleakalā National Park, 1920 m, 1977, L. Stemmermann 2224 (HAW); Maui, East Maui, Haleakalā National Park, 1982, A. Medeiros 313 (HAW); Maui, East Maui, Haleakalā National Park, Kipahulu Valley, 2100 m, 1984, A. Medeiros & D Miranda 314 (HAW); Maui, East Maui, Haleakalā National Park, Paliku, C. W. Smith 2163, 3098 (HAW); Maui, East Maui, Kipahulu Forest Reserve, 1995 m, 1976, P. K. Higashino & G. Mizuno 326, 1719 (HAW); Maui, East Maui, Wainapanapa, 2060 m, 1977, L. Stemmermann 2450 (HAW); Maui, East Maui, Haleakalā Volcano, Haleakalā National Park, Paliku, 1950 m, 1975, W. J. Hoe 3761 (HAW); Maui, East Maui, Haleakalā Volcano, Lower Waikamoi Preserve, 20°48′23″N, 156°15′19″W, 1200–1300 m, 2013, B. Moncada, R. Lücking & P. Bily 6946 (F), 6950 (B, F); Maui, East Maui, Haleakalā Volcano, Upper Waikamoi Preserve, 20°46′07″N, 156°14′17″W, 1800–2100 m, 2013, B. Moncada, R. Lücking & P. Thomas 6977 (F, HAW), 6980 (B), 6981 (F), 6989 (F), 6991 (B, F, HAW), 6992 (B), 6993 (F), 6996 (B, F, HAW), 7001 (HAW); Maui, Kipahulu Valley, 520 m, 1980, G. Y. Daida 756 (HAW); Maui, Olinda, Koolau Forest Reserve, 20°49′23″N, 156°16′12″W, 910 m, 1992, R. Rosentreter 8086b, 8094, 8107 (SRP); Maui, Olinda Koolan Forest Reserve, 20°49′40″N, 156°14′10″W, 1280 m, 1992, T. H. Nash III 42205 (ASU, MIN, WIS); Maui, Waikamoi Nature Conservancy Reserve, 20°46′12″N, 156°14′48″W, 2073 m, 1992, R. Rosentreter 8129b (F, SRP); Molokai, Molokai Forest Reserve, 1975, P. K. Higashino 460 (HAW); Oahu, Koolau Mountains, 1970, C. W. Smith 130f (HAW); Oahu, Waianae Mountains, 1220 m, on scrub Metrosideros, 1985, C. W. Smith 8421 (HAW); Oahu, Waianae Mountain Range, Kaua Trail, 945 m, 1978, G. Y. Daida 201 (HAW); Oahu, Kaala, C. W. Smith 1742 (HAW); Oahu, Waahila Ridge Trail, 1979, C. S. Futa 12 (HAW).
Sticta waikamoi Moncada & Lücking
Moncada et al., Pl. Fung. Syst. 65, 114 (2020); type: USA, Hawaii, Maui, East Maui, Haleakalā Volcano, Upper Waikamoi Preserve, 20°46′07″N, 156°14′17″W, 1800–2100 m, 2013, B. Moncada, R. Lücking & P. Thomas 7000 (F—holotype!; B, HAW—isotypes!).
Primary photobiont cyanobacterial (Nostoc). Stipe absent. Thallus monophyllous, irregularly orbicular, to 7 cm across, anisotomously branched; lobes 4–7 mm broad, elongate to narrowly flabellate, more or less horizontal, not usually overlapping, involute to shallowly canaliculate, their margins entire to broadly crenulate. Upper surface even, olive-grey to grey-brown when fresh, light yellowish to greyish brown when dry, glabrous, without papillae or pruina, without distinct maculae; marginal cilia not differentiated but lower tomentum often projecting beyond the margins to resemble short, brown-black cilia. Isidia present, predominantly marginal, to 0.5 mm long and broad, grey-brown, somewhat shiny, usually somewhat flattened, branched and becoming coralloid. Lower surface slightly uneven, dark brown. Primary tomentum thick and dense, forming dark brown, arachnoid tufts of fasciculate hyphae; hairs 100–300 μm long, in fascicles of 10–20, mostly unbranched but strongly agglutinate, dark brown, cylindrical hyphae with free apices. Secondary tomentum not discernible except in microscopic sections; hairs 20–30 μm long, comprising single, branched, weakly moniliform hyphae with somewhat inflated cells and free apices. Rhizines present, scattered towards the thallus centre. Cyphellae rather dense, 21–60 per cm2, rounded, plane, immersed to becoming erumpent, white to cream, strongly contrasting with the dark tomentum; pore 0.3–1(–1.5) mm diam.; cells of basal membrane irregularly bulging but lacking papillae.
Apothecia not observed.
Secondary chemistry
No substances detected by TLC; medulla K− to slowly K+ faintly yellowish, cyphellae K+ slowly ochraceous.
Distribution and ecology
Sticta waikamoi is so far known only from a small number of collections, at mid to high altitudes between 1585 and 2195 m, including mixed conifer forest on the island of Maui. While this type of forest conveys the impression of being rather undisturbed, it is not native to Hawaii, since all conifers have been introduced to the archipelago. While the type was growing on the ground over bryophytes, the species may occasionally also be epiphytic, then usually over bryophyte mats.
Remarks
Together with Sticta andina, S. waikamoi corresponds best to what has been traditionally identified with the name S. weigelii. However, the latter is a neotropical taxon and not directly related (Moncada et al. Reference Moncada, Lücking and Lumbsch2020). Sticta weigelii s. str. also deviates in its thinner lower tomentum and the often yellow cyphellae (Galloway Reference Galloway2006; Moncada Reference Moncada2012). Sticta andina differs in the generally narrower lobes and lack of rhizines and typically grows epiphytically. Sticta waikamoi is closely related to S. rhizinata but differs in several substitutions in the ITS barcoding marker and is much smaller overall, with less conspicuous rhizines (Moncada & Lücking Reference Moncada and Lücking2012; Moncada et al. Reference Moncada, Lücking and Lumbsch2020).
Additional specimens examined
USA: Hawaii: Hawaii, Mauna Loa, Pohakuloa training area, 1585 m, 1977, P. K. Higashino 323 (HAW); Maui, East Maui, Makawao Forest Reserve, 1800 m, 1975, C. W. Smith 1950a (HAW); Maui, Haleakalā, N slope, Frisbee meadow above Poouli Flats, 2195 m, 1987, C. W. Smith 8455 (HAW).
Revised taxonomic concept and excluded names
Several names previously reported in the genus Sticta for Hawaii must be excluded from the Hawaiian lichen biota, including at least one name from digital online repositories. We also performed an analysis of the specimens housed in HAW to see how previously applied names correspond to the revised taxonomy (Fig. 3). Thus, the two common species confirmed to be present in Hawaii, S. fuliginosa and S. tomentosa, were largely identified correctly but some specimens also corresponded to other taxa, including S. antoniana and S. scabrosa var. hawaiiensis in the case of S. tomentosa and S. hawaiiensis and S. scabrosa var. hawaiiensis in the case of S. fuliginosa. Specimens previously identified as S. weigelii represented no less than seven different taxa, mostly S. andina and S. scabrosa subsp. hawaiiensis. Sticta plumbicolor was mostly correctly identified but some specimens corresponded to S. andina and to the newly recognized S. flynnii and S. smithii (Fig. 3).
Sticta ambavillaria (Bory) Ach. — Not present in Hawaii; specimens identified with this name represent S. antoniana (Moncada et al. Reference Moncada, Lücking and Lumbsch2020).
Sticta babingtonii D. J. Galloway — The name of this green-algal species was applied to an unpublished herbarium collection from Maui (Olinda, Koolau Forest Reserve North Haleakala, 910 m, 1992, R. Rosentreter 8108 (SRP)), reported in the Lichen Portal of the Consortium of North American Lichen Herbaria (CNALH): [https://lichenportal.org/cnalh/collections/individual/index.php?occid=1577932&clid=0]. Revision of a digital image of the specimen revealed it to be S. andina.
Sticta cyphellulata (Müll. Arg.) Hue — Not present in Hawaii; specimens identified with this name represent S. smithii and probably also S. flynnii (Moncada et al. Reference Moncada, Lücking and Lumbsch2020).
Sticta filix (Sw.) Nyl. — Not present in Hawaii; an erroneous report of uncertain origin (Moncada et al. Reference Moncada, Lücking and Lumbsch2020).
Sticta weigelii (Ach.) Vain. — Not present in Hawaii; specimens identified with this name represent S. andina, S. scabrosa subsp. hawaiiensis and S. waikamoi (Moncada et al. Reference Moncada, Lücking and Lumbsch2020).
Sticta beauvoisii Delise; S. weigelii var. beauvoisii (Delise) Hue — Not present in Hawaii; specimens identified with this name represent S. scabrosa subsp. hawaiiensis (Moncada et al. Reference Moncada, Lücking and Lumbsch2020).
Sticta weigelii var. lutescens (Taylor) H. Magn. — Not present in Hawaii; specimens identified with this name probably represent S. scabrosa subsp. hawaiiensis (Moncada et al. Reference Moncada, Lücking and Lumbsch2020).
Sticta weigelii var. peruviana (Delise) Vain. — Not present in Hawaii; specimens identified with this name probably represent S. scabrosa subsp. hawaiiensis (Moncada et al. Reference Moncada, Lücking and Lumbsch2020).
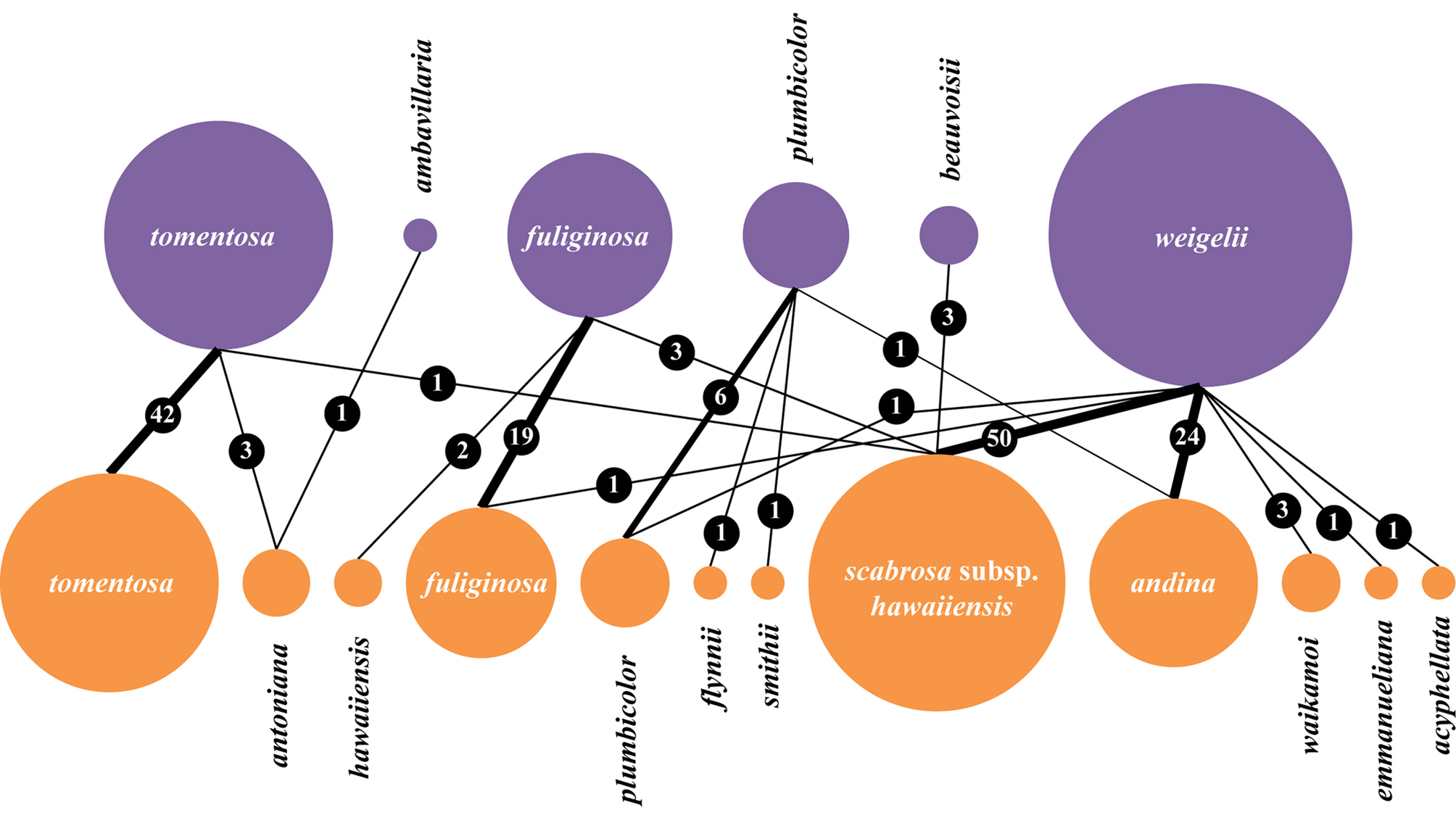
Fig. 3. Correspondence of identifications of Sticta lichens in HAW under the previous (above, purple) and the revised taxonomic concept (below, orange). The size of the circles is proportional to the number of specimens corresponding to a given name. The number of specimens corresponding to a particular ‘pair’ of identifications ‘before’ vs. ‘after’ is also indicated. In colour online.
Discussion
As outlined above and by Moncada et al. (Reference Moncada, Mercado-Díaz, Smith, Bungartz, Sérusiaux, Lumbsch and Lücking2021), eight species of Sticta had previously been listed for Hawaii: S. ambavillaria, S. beauvoisii, S. cyphellulata, S. filix, S. fuliginosa, S. plumbicolor, S. tomentosa and S. weigelii (Elix & McCarthy Reference Elix and McCarthy1998, Reference Elix and McCarthy2008; Smith Reference Smith2013). One additional species, S. limbata, was listed in an ecophysiological study (Benner & Vitousek Reference Benner and Vitousek2012). Of these, only three widespread taxa (S. fuliginosa, S. limbata, S. tomentosa) and one putatively endemic species (S. plumbicolor) could be confirmed as a result of a molecular phylogenetic revision (Moncada et al. Reference Moncada, Mercado-Díaz, Smith, Bungartz, Sérusiaux, Lumbsch and Lücking2021). Of the 13 species now recognized, one was a new record (S. andina) and eight were taxa new to science (seven species and one subspecies). Thus, the overlap in names recorded for Hawaii before and after this revision is only 38%. This substantial change in the taxonomic delimitation of Hawaiian Sticta has implications for other studies of these lichens, including their potential applications, for example as environmental monitors.
For example, Czeczuga et al. (Reference Czeczuga, Harada and Smith1997) analyzed carotenoid content in various lichens including S. weigelii from Hawaii. No voucher information was given but the locality data (Oahu, Melaleuca) indicate that the studied material probably represented S. scabrosa subsp. hawaiiensis, which is common in that area. Hagiwara (Reference Hagiwara2016) and Hagiwara et al. (Reference Hagiwara, Wright, Tabandera, Kelman, Backofen, Ómarsdóttir and Wright2016) analyzed antioxidant and antitumoral properties in a range of lichens collected in Hawaii and Iceland, including Pseudocyphellaria hawaiiensis H. Magn., P. xanthosticta (Pers.) Moncada & Lücking, and Sticta weigelii. While the taxonomy of Pseudocyphellaria followed the earlier revision by Moncada et al. (Reference Moncada, Reidy and Lücking2014a), the material identified as S. weigelii is likely to correspond to S. scabrosa subsp. hawaiiensis, because the Hawaiian lichens in that study had been collected at low altitudes (0–500 m).
Benner et al. (Reference Benner, Conroy, Lunch, Toyoda and Vitousek2007), Benner (Reference Benner2011) and Benner & Vitousek (Reference Benner and Vitousek2012) investigated the correlation between phosphorus, nitrogen and cyanolichens in Hawaiian forests, including experimental P fertilization. Lobarioid species included in these studies were Crocodia aurata (Ach.) Link (as Pseudocyphellaria), Lobariella crenulata (Hook.) Yoshim., P. crocata (L.) Vain., Sticta beauvoisii, S. fuliginosa, S. limbata, S. tomentosa and S. weigelii. The presence of L. crenulata in Hawaii has not been confirmed by molecular data and the studied material may represent the newly recognized L. sandwicensis Lücking et al. (Lücking et al. Reference Lücking, Moncada and Smith2017a). Crocodia aurata indeed occurs in Hawaii (Moncada et al. Reference Moncada, Reidy and Lücking2014a). However, Lobariella and Crocodia are chlorolichens and it is unclear why they were classified as cyanolichens, with rather high rates of N-fixation (Benner & Vitousek Reference Benner and Vitousek2012), raising doubt about the underlying species identifications. Pseudocyphellaria crocata s. str. is absent from the Hawaiian archipelago and the material studied by Benner et al. (Reference Benner, Conroy, Lunch, Toyoda and Vitousek2007) and Benner & Vitousek (Reference Benner and Vitousek2012) refers to one or several newly distinguished taxa (Moncada et al. Reference Moncada, Reidy and Lücking2014a). In the absence of voucher material, it is impossible to ascertain which species were studied. In the case of the five representatives of Sticta, the material identified as S. beauvoisii is likely to represent S. scabrosa subsp. hawaiiensis, whereas S. weigelii could well refer to S. andina. The identifications of the other three taxa (S. fuliginosa, S. limbata, S. tomentosa) appear to be correct. Notably, Benner & Vitousek (Reference Benner and Vitousek2012) found that the forest plot fertilized with P showed an increased abundance of lobarioid macrolichens, including species of Sticta. Between the two species of Sticta specifically tested for N-fixation, S. tomentosa showed higher N-fixation in the non-fertilized control plot, whereas for S. beauvoisii (= S. scabrosa subsp. hawaiiensis) it was higher in the P-fertilized plot. This result can be viewed in a new light, given the finding that among Hawaiian Sticta, S. tomentosa is largely confined to undisturbed forests (see also Smith Reference Smith and Galloway1991), whereas S. scabrosa subsp. hawaiiensis is frequent in disturbed and anthropogenic vegetation.
These examples show that revised species taxonomies also challenge the results of studies in ecology, ecophysiology, biogeography, biochemistry and other fields of research, a largely overlooked problem. Due to the frequent lack of voucher citations, correct identification of taxa used in such studies can often not be ascertained. It is therefore of the utmost importance that such studies deposit voucher material in public herbaria in the same way as taxonomic works, preferably with accessible digital information including specimen imagery. Collaboration with experts or DNA barcoding may improve the taxonomic component of such studies (e.g. Paudel et al. Reference Paudel, Bhattarai, Lee, Hong, Shin and Yim2008; Plaza et al. Reference Plaza, de Torres, Lücking, Vizcaya and Medina2014; Bajpai et al. Reference Bajpai, Shukla, Singh, Rana and Upreti2015; Xu et al. Reference Xu, Heidmarsson, Olafsdottir, Buonfiglio, Kogej and Ómarsdóttir2016), but this does not replace proper deposition and citation of voucher material. DNA barcoding in particular is also often misinterpreted. In a study on physiological and genetic effects of chromium on thalli of Pyxine cocoes in India (Bajpai et al. Reference Bajpai, Shukla, Singh, Rana and Upreti2015), the authors used ITS barcodes to support the identification of their material. However, our own revision of ITS sequences available for P. cocoes revealed that it forms three distinct clades: one neotropical clade (KX512936), sister to a clade including a subclade formed by two specimens from Singapore and China (AF540540, KY611874), and another subclade formed by the Indian specimens from the above study (KF691782, KF691783, KF691784, KF691785, KF691786, KF691787). Based on the underlying topology and the large number of substitutions in the ITS barcoding marker, these clades represent three different species. Yet, even with these data to hand, the authors did not provide an adjusted taxonomy and identified their material uncritically with the name P. cocoes.
Another important aspect of revised and refined species taxonomies is the use of lichens in environmental monitoring. In a study of the Sticta filix group in New Zealand, Ranft et al. (Reference Ranft, Moncada, de Lange, Lumbsch and Lücking2018) showed that two previously unrecognized species in this group had a higher fidelity for undisturbed forest vegetation than the two species previously distinguished based on a broader approach to species delimitation. Given the particular situation in Hawaii as a threatened island biota (Kirch Reference Kirch1982; Stone & Scott Reference Stone and Scott1985; Stone et al. Reference Stone, Smith and Tunison1992; Sakai et al. Reference Sakai, Wagner and Mehrhoff2002; Department of Land and Natural Resources 2007; US Fish and Wildlife Service 2010; Wood Reference Wood2012), the disparate ecology of Hawaiian Sticta enables the use of these lichens to monitor ecosystem health. This approach is further facilitated by the possibility of identifying the currently recognized taxa in the field. Nine of the species now recognized appear to be confined to closed or more or less undisturbed forest (Table 1). As ‘undisturbed’ one may thereby also classify non-native old-growth forest such as the introduced conifer forest on Maui. On the other side of the spectrum is the widespread and somewhat weedy S. scabrosa subsp. hawaiiensis. Sticta fuliginosa is somewhat intermediate in its ecological requirements, being found in undisturbed forest but also disturbed sites. These observations would allow the establishment of a protocol using Sticta species along a disturbance gradient (Table 1) (1 = least disturbed, 5 = most disturbed), using an abundance-weighted environmental monitoring score E based on relative species abundance at a given monitoring site:
where Ei = individual species score (see Table 1) and Ai = individual relative species abundance (e.g. using a grid system or other sampling design). For example, a site with S. scabrosa subsp. hawaiiensis (90% abundance) and S. fuliginosa (10%) would give a weighted score of E = 5 × 0.9 + 3 × 0.1 = 4.8 (highly disturbed). In contrast, a site with S. andina (30%), S. flynnii (5%), S. fuliginosa (10%), S. smithii (5%) and S. tomentosa (50%) would result in a weighted score of E = 1 × 0.3 + 1 × 0.05 + 3 × 0.1 + 2 × 0.05 + 1 × 0.5 = 1.25 (less disturbed).
Table 1. Proposed environmental scores (1 = least disturbed, 5 = most disturbed) for currently distinguished species of Hawaiian Sticta to be used in a simple monitoring protocol to assess ecosystem health.

Traditional taxonomy of the genus Sticta in Hawaii would not have allowed such an approach, as for instance the ecologically disparate S. andina and S. scabrosa subsp. hawaiiensis or S. hawaiiensis and S. fuliginosa had been subsumed under the names S. weigelii and S. fuliginosa, respectively. This impact of accurate taxonomy on the use of lichens as biomonitors was also discussed in a study of the S. filix group in New Zealand (Ranft et al. Reference Ranft, Moncada, de Lange, Lumbsch and Lücking2018).
Conceivably, a next step following these results would be a conservation assessment of Sticta lichens in Hawaii. However, since this is the first study that offers an accurate taxonomic treatment of the species, we consider it premature to carry out such an assessment with the current data. Instead, we hope this treatment encourages researchers to study the genus in more detail and with quantitative approaches, including the protocol proposed above. The small species especially need to be looked for more extensively, since these are likely to have been overlooked in the past or misinterpreted as young specimens not worth collection. It is therefore difficult to state whether these species are genuinely rare. Among the conspicuous taxa, S. fuliginosa and S. scabrosa subsp. hawaiiensis are certainly of the least concern, as they seem to tolerate various degrees of disturbance. Also S. tomentosa, although mostly found in undisturbed forest, appears to be abundant where forest vegetation is properly protected.
Acknowledgements
Funding for this study was provided by the National Science Foundation (NSF) to The Field Museum: ‘ATM– Assembling a Taxonomic Monograph: The Lichen Family Graphidaceae’ (DEB-1025861; PI HTL, CoPI RL) and ‘Collaborative Research: Evolution, Diversification, and Conservation of a Megadiverse Flagship Lichen Genus’ (DEB-1354884; PI HTL, CoPI RL). Further financial support came from The Field Museum's Women's Board Field Dreams program 2011, through gifts by the Robert Thomas Bobins Foundation through Mrs Virginia Bobins (Chicago), Mr and Mrs John Borland Jr. (Chicago), Mrs Peggy Carr (Chicago) and Mrs Sue Dickes (Winnetka). Patrick Bily (The Nature Conservancy Hawaii), Timothy Flynn (National Tropical Botanical Garden), Daniel Pomaika'i (Maui Soil and Water Conservation Districts, Maunalei Arboretum) and Philip Thomas (Research Corporation of the University of Hawaii and the Hawaiian Ecosystems at Risk Project) assisted with fieldwork on Maui and Kauai. Jill Kajikawa-Kent (University of Hawaii) and Rae Matthews and the Clark family (National Tropical Botanical Garden) also provided logistical support. The Hawaii Department of Land and Natural Resources, Divisions of Forestry and Wildlife and Division of State Parks, provided collecting and research permits, and Chelsea Carineo, Wendee Kokubun, Ryan Peralta, Patrick Porter, Matthew Rittenhouse and Lancede Silva are thanked for processing the corresponding requests. Roger Rosentreter kindly provided information on and digital images of Hawaiian specimens identified as Sticta babingtonii.
Author ORCIDs
Bibiana Moncada, 0000-0001-9984-2918; Robert Lücking, 0000-0002-3431-4636.







