INTRODUCTION
Humpback whales (Megaptera novaeangliae, Borowski 1781) have characteristic temporal and spatial migratory patterns that enable the species to take advantage of the great productivity of high latitude waters for feeding during the austral summer, and breeding and calving in low latitudes during the winter months. Winter coastal distribution (Dawbin, Reference Dawbin1956; Clapham, Reference Clapham, Mann, Connor, Tyack and Whitehead2000) associated with islands and reef systems (Clapham, Reference Clapham, Perrin, Würsig and Thewissen2009) is common in many humpback whale populations. Females with calves occur even closer to shore in shallower and more protected waters (Whitehead & Moore, Reference Whitehead and Moore1982; Smultea, Reference Smultea1994; Ersts & Rosenbaum, Reference Ersts and Rosenbaum2003). Low latitude warmer waters (Clapham, Reference Clapham, Mann, Connor, Tyack and Whitehead2000) with low predation risk (Corkeron & Connor, Reference Corkeron and Connor1999) are thought to enhance the chances of survival of humpback whale newborn calves.
The population that migrates to the Brazilian coast between July and November (Martins et al., Reference Martins, Morete, Engel, Freitas, Secchi and Kinas2001) is part of Breeding Stock A (IWC, 2005). This population feeds off South Georgia and Sandwich Islands (Zerbini et al., Reference Zerbini, Andriolo, Heide-Jørgensen, Pizzorno, Maia, VanBlaricom, DeMaster, Simões-Lopes, Moreira and Bethlem2006, Reference Zerbini, Andriolo, Heide-Jørgensen, Moreira, Pizzorno, Maia, VanBlaricom and DeMaster2011; Engel & Martin, Reference Engel and Martin2009) which are ~4000 km distance from this breeding ground (Stevick et al., Reference Stevick, Godoy, McOsker, Engel and Allen2006).
The number of humpbacks that migrate to Brazil is increasing (Freitas et al., Reference Freitas, Kinas, Martins and Engel2004; Andriolo et al., Reference Andriolo, Kinas, Engel, Martins and Rufino2010; Bortolotto et al., Reference Bortolotto, Danilewicz, Andriolo, Secchi and Zerbini2016). For years, all research efforts were focused in the Abrolhos region (Martins et al., Reference Martins, Morete, Engel, Freitas, Secchi and Kinas2001; Morete et al., Reference Morete, Pace, Martins, Freitas and Engel2003), which continues to be the main breeding area (Andriolo et al., Reference Andriolo, Martins, Engel, Pizzorno, Más-Rosa, Freitas, Morete and Kinas2006; Reference Andriolo, Kinas, Engel, Martins and Rufino2010). However, the species occurs along the entire north-eastern coast of Brazil (Zerbini et al., Reference Zerbini, Andriolo, Rocha, Simões-Lopes, Siciliano, Pizzorno, Waite, DeMaster and VanBlaricom2004; Lunardi et al., Reference Lunardi, Engel and Macedo2008) and the population shows a significant expansion northern of Abrolhos, reoccupying winter areas (Rossi-Santos et al., Reference Rossi-Santos, Neto, Baracho, Cipolotti, Marcovaldi and Engel2008; Andriolo et al., Reference Andriolo, Kinas, Engel, Martins and Rufino2010) used before the whaling period (Morais et al., Reference Morais, Danilewicz, Zerbini, Edmundson, Hart and Bortolotto2016). Surveys carried out between 2002 and 2005 showed a gradual increase in the Brazilian population reaching 6404 individuals in 2005 (Andriolo et al., Reference Andriolo, Kinas, Engel, Martins and Rufino2010). Bortolotto et al. (Reference Bortolotto, Danilewicz, Andriolo, Secchi and Zerbini2016) had estimated 19,429 humpback whales in 2012, while Pavanato et al. (Reference Pavanato, Wedekin, Guilherme-Silveira, Engel and Kinas2017) had estimated 12,123 individuals in 2015 using different methodologies in a larger study area. The IUCN (International Union for the Conservation of Nature) has changed the species status from ‘vulnerable’ to ‘least concern’ (IUCN, 2013) due to the increase in size of most humpback whale populations worldwide.
Zerbini et al. (Reference Zerbini, Andriolo, Rocha, Simões-Lopes, Siciliano, Pizzorno, Waite, DeMaster and VanBlaricom2004) surveyed the north-east of Brazil and found most humpback whale sightings to occur within 50 m depth, which normally is associated with proximity to the coastline. In Brazil, previous studies mainly occurred in two regions along the state of Bahia: (1) the Abrolhos Bank located off the southern limit of the state and considered to be a unique environment compared with other regions along the coast and (2) Praia do Forte to the north. There is a gap of knowledge about the species between these two regions, where our study site is located, between Itacaré and Ilhéus, and where few human activities currently occur. The region is unexplored except for a few boat-based and aerial scientific surveys (Rossi-Santos et al., Reference Rossi-Santos, Neto, Baracho, Cipolotti, Marcovaldi and Engel2008; Andriolo et al., Reference Andriolo, Kinas, Engel, Martins and Rufino2010; Baracho-Neto et al., Reference Baracho-Neto, Neto, Rossi-Santos, Wedekin, Neves, Lima and Faria2012). Between 2002 and 2005, during aerial surveys aimed at estimating the humpback whale population along the Brazilian coast, the Itacaré/Ilhéus region presented densities between 0.010 and 0.026 individuals per km2 while over the Abrolhos Bank densities were between 0.028 and 0.091 individuals per km2 (Andriolo et al., Reference Andriolo, Kinas, Engel, Martins and Rufino2010).
The presence of whales close to shore and the shoreline features that include an elevated point (Serra Grande), allowed observations from a land-based station (Würsig et al., Reference Würsig, Cipriano, Würsig, Pryor and Norris1991). This research methodology has been applied to study humpback whales for two decades in the Abrolhos archipelago (Morete et al., Reference Morete, Pace, Martins, Freitas and Engel2003, Reference Morete, Bisi, Pace and Rosso2008), which is located within the Abrolhos Marine National Park. Our aim in this study was to characterize patterns of relative abundance and habitat use throughout the winter season in Itacaré/Ilhéus region from a land-based station located at Serra Grande. Social strategies used during the reproductive season and other unknown aspects of humpback whale distribution in this region will provide information to support better habitat protection and other management decisions.
MATERIALS AND METHODS
Study site
Data were collected from the highest point of Serra Grande (14°28′30″S 39°01′50″W), ~34 km north from the city of Ilhéus, southern Bahia state, north-eastern Brazil (Figure 1). The land-based station is located 315 m from the coastline at an elevation of 93 m above mean sea level. We considered a radius of 14 km from the observation point to define the study area between 70 and 184° (True) covering 195.63 km2. The orientation of the coastline, and the existence of vegetation and rocks prevent the monitoring of the north-east of the area.
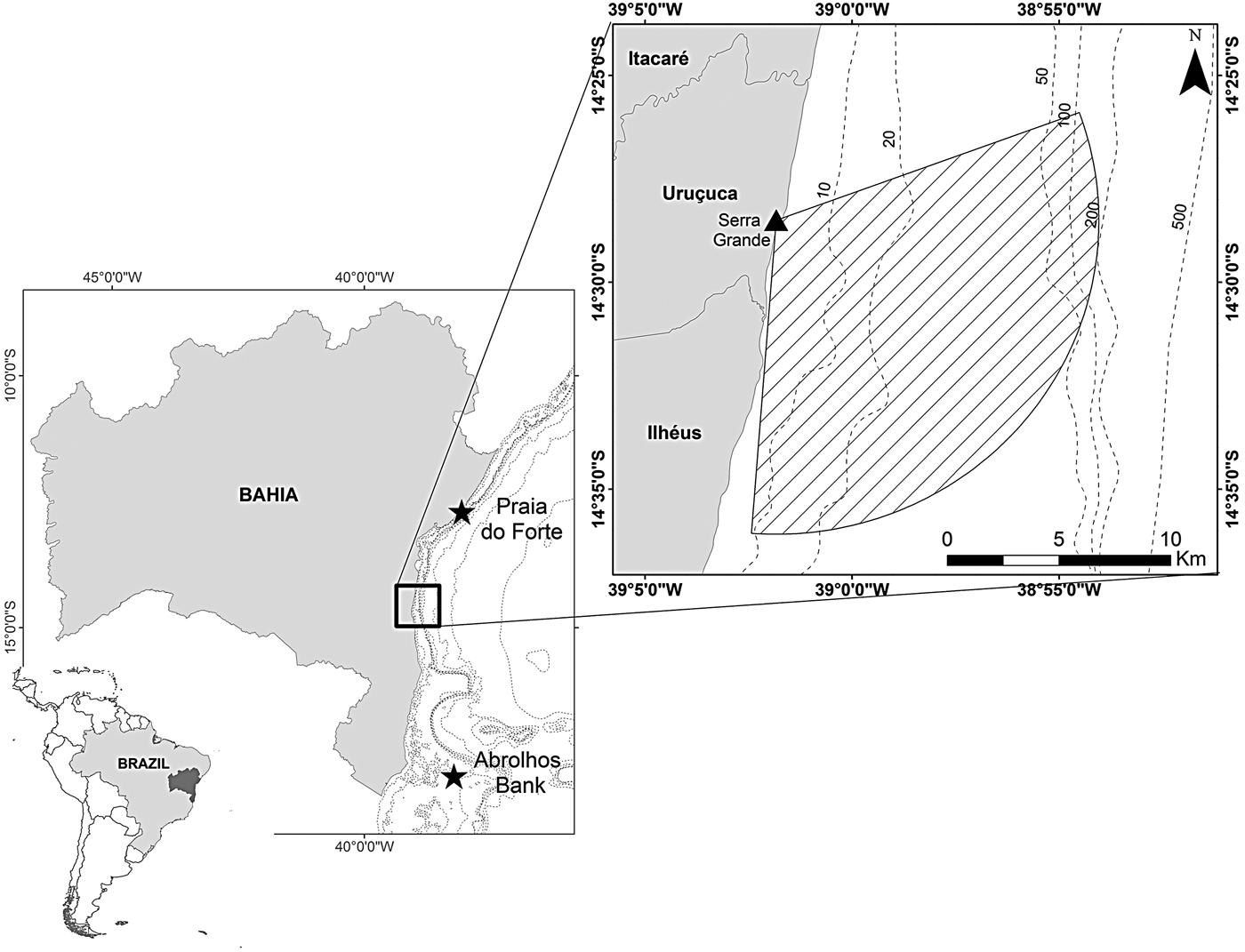
Fig. 1. Serra Grande study site located in north-eastern Brazil where a land-based observation station at elevation 93 m was used to conduct visual surveys that covered an area of 195.63 km2 (striped area).
The region's ocean floor is predominantly made of rocks and sand (Freire & Dominguez, Reference Freire and Dominguez2006). Mean water temperature varies during the year between 24 and 29°C (NOAA, 2016).
Visual surveys
Observations were made between July and October in 2014 and 2015. Data collection was conducted during the daytime between 07:20 and 16:05 h following survey methods described by Mann (Reference Mann1999), each survey being of 1 h duration (Morete et al., Reference Morete, Bisi and Rosso2007, Reference Morete, Bisi, Pace and Rosso2008). Morning and afternoon surveys were undertaken when weather conditions allowed good visibility of the skyline and when sea state was equal or below Beaufort 4. The mean interval between surveys was 3.22 h (SD = 0.68) allowing for observed groups to have moved away by the time of the second survey, leading to sample independence (Frankel & Clark, Reference Frankel and Clark2002).
Each survey was conducted by two or three dedicated observers and active search done with naked eye and binoculars 7 × 50. Whales were located based on presence cues such as blows, water splash from aerial behaviours, or exposure of a body part (Morete et al., Reference Morete, Bisi, Pace and Rosso2008). When a group of whales was sighted, the main observer (same person throughout the study) tracked and monitored the group using a total station TOPCON ES105 with 5′ of precision and 30-power monocular magnification until the location angle, size, composition and behaviour of the group was identified (Morete et al., Reference Morete, Bisi, Pace and Rosso2008). Meanwhile, the other observers kept monitoring the area, searching for other whale groups.
In order to avoid counting groups twice, if there was any doubt about the discrimination of sighted groups during a survey, the effort was interrupted and the ongoing survey would be cancelled and another one started (Morete et al., Reference Morete, Bisi, Pace and Rosso2008). At the start and end of each survey and any time that conditions changed, the wind direction, cloud cover and Beaufort Sea state were registered by the main observer.
Definitions
A group was defined as a single or several individuals moving in coordination towards the same general direction no more than 100 m apart from each other (Whitehead, Reference Whitehead1983; Morete et al., Reference Morete, Bisi, Pace and Rosso2008).
Group composition categories were defined as (a) mother with calf (MOC), (b) mother and its calf accompanied by an escort (MOCE), (c) mother and its calf accompanied by two or more escorts (MOCE/+), and in the absence of a calf, group definitions were based on the number of individual whales, (d) solitary (1AD), when a lone adult was observed, (e) two adults (dyad) or (f) more than two adults (multiple) (Morete et al., Reference Morete, Bisi and Rosso2007; Dunlop et al., Reference Dunlop, Cato and Noad2008). When it was not possible to determine the composition, the group was identified as ‘undetermined’. The distance to the sightings did not allow the discrimination of juveniles, therefore we considered only two age classes: adults and calves, the latter identified as such when its length was up to 50% that of an adult (Chittleborough, Reference Chittleborough1953).
Spatial analyses
The total station TOPCON ES105 allows measurement of horizontal angles between two points, a known reference point and the target object, and also the vertical angle between the target object and the observer (Gailey & Ortega-Ortiz, Reference Gailey and Ortega-Ortiz2002; Bailey & Lusseau, Reference Bailey and Lusseau2004). Total station and reference point Universal Transverse Mercator (UTM) coordinates were determined by GNSS (Global Navigation Satellite System) positioning, with a precision of 1.00 mm. Orthometric altitudes of these points were determined using Geoidal MAPGEO 2010 model (Monico, Reference Monico2008). These point locations added to the height of the installed total station and tidal variation allowed calculations of UTM (E, N) coordinates of all points measured using trigonometric equations (Gonçalves, Reference Gonçalves2017). Errors due to Earth curvature (Vanicek & Krakiwsky, Reference Vanicek and Krakiwsky1996) were corrected by transforming the horizontal distances to spherical distances.
Depth at the locations where groups were sighted were obtained by ArcGIS 9.3 Extraction tool of the Spatial Analyst using bathymetric information constructed from vectorization of nautical charts 1200 and 2105 from the Brazilian Navy (CHM, 2011–2015) followed by interpolation of depth values using ordinary kriging geostatistical analyses (Childs, Reference Childs2004). Distance to coastline was calculated through the distances between the meridians of the position of the sighted group and the coast using Google Earth in order to acquire more precise values given the high resolution mapping and detailed images of the coast.
Statistical analyses
GROUPS
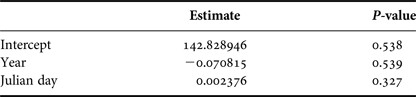
In order to examine how group size varied in the area throughout the season, we considered only the data from groups for which size and composition were determined with confidence. A generalized linear model (GLM) was used to fit the group size data into a Poisson distribution. Year and Julian day were used as predictors of group size.
RELATIVE ABUNDANCE
Because of the fluctuation of whale relative abundance between seasons (Morete et al., Reference Morete, Bisi, Pace and Rosso2008), the peak of each season was calculated using a segmented regression (Muggeo, Reference Muggeo2008) of the whale counts per survey. The seasons were divided into three periods (initial, middle and final) within a calving season of 123 days, each period having 41 days (Morete et al., Reference Morete, Bisi and Rosso2007), and the peak of the season being the centre of the middle period, which varied depending on the year. Due to the lack of normality of the distribution, we used a Mann–Whitney U test to verify if hourly whale counts changed between the sampled years (2014 and 2015). A GLM was used to fit the number of whales sighted per hour (number of adults and calves separately) into a Poisson distribution and test if it changed as the season progressed. The model to explain adult relative abundance included year and lunar phase (four categories considered by NOAA) as categorical predictors and Julian days and sea state (Beaufort 1 to 4) as continuous predictor variables, as well as the interaction between the variables: year and Julian days. The model to explain calf abundance also included number of adults as a predictor variable. The number of individuals considered in undetermined groups was the maximum number of sighted animals to avoid underestimation of the total number of whales in such cases. The residuals and the residual variation were verified to ensure that the models were adequate with respect to the premises.
HABITAT USE
An ANOVA followed by a Tukey's honest significant difference (HSD) test was used to verify if a whale group's mean distance to coast and depth were different among periods of the season. Spatial distribution of groups in the sampled area along the season was mapped as Kernel densities using Hawth's Tools developed as an extension of ArcGIS (Beyer, Reference Beyer2004). We used default values for the parameters within this tool and the band (h) was defined as 1.0 km to smooth over 100 × 100 m surface cell size using the normal bivariate method. For comparison of the maps among the different periods, the values were normalized to a common scale (0–1). Statistical transformation was applied on a logarithmic function that rescaled the values maintaining the original form of distribution. We used t tests to establish whether distance to coast and depth were different between groups with and without calves. Within groups with calves, such differences were tested between MOC, MOCE and MOCE/+ using ANOVA. Mother and calf groups (MOC) were defined as the baseline to verify differences with MOCE and MOCE/+. We did not find any significant deviations from normality given the robustness of the analyses to deviations from this assumption. Variances were also assumed to be equal in all ANOVAs except those used to test depth differences between groups with calves. In those cases, we used ANOVAs with Welch's correction for unequal variances. All statistical analyses were run in R 3.0.2 (R, Development Core Team) with the significance value (α) of 0.05.
RESULTS
Ninety-three hours of surveys (Table 1) were carried out during 67 days in the field (37 days in 2014, and 30 in 2015). The identification of the number of individuals and age class (adult or calf) in the groups was possible for 146 (67.59%) out of the 216 groups sighted. Adult individuals were the majority (N = 278) compared with calves (N = 31).
Table 1. Number of surveys performed with the number of field days in parentheses by period of the season from a land-based observation station in 2014 and 2015 in Serra Grande, Bahia state, Brazil.

Groups
Group size varied from a single individual to five whales. Mean group size was 2.12 (SD = 0.96). Year and day of the season did not affect group sizes (Table 2).
Table 2. Generalized Linear Model (Poisson distribution) parameter estimates and P-values for year and Julian day that explained group sizes of humpback whales observed from a land-based observation station in 2014 and 2015 in Serra Grande, Bahia state, Brazil.
The most common group composition was dyad 32.9% (N = 48), followed by solitary individuals 26.7% (N = 39) and groups with calves 21.2% (N = 31). Multiple groups were the least common in the area (19.2%, N = 28). Groups with a calf were comprised mostly of MOC (61.3%, n = 19), MOCE (22.6%, N = 7), and MOCE/+ (16.1%, N = 5) categories.
Relative abundance
Abundance in both 2014 and 2015 seasons was characterized by a segmented distribution with the break point between the end of August and beginning of September (Figure 2). The peak for 2014 was 23 August and for 2015, 4 September. The segmented regression model was significant (P < 0.001) and the regression coefficient was positive for the first half and negative for the second half. Adult hourly abundance pooled for both years varied from 0 to 14 and calves from 0 to 4 individuals. The maximum hourly abundance (17 individuals) was observed in the beginning of September 2015.
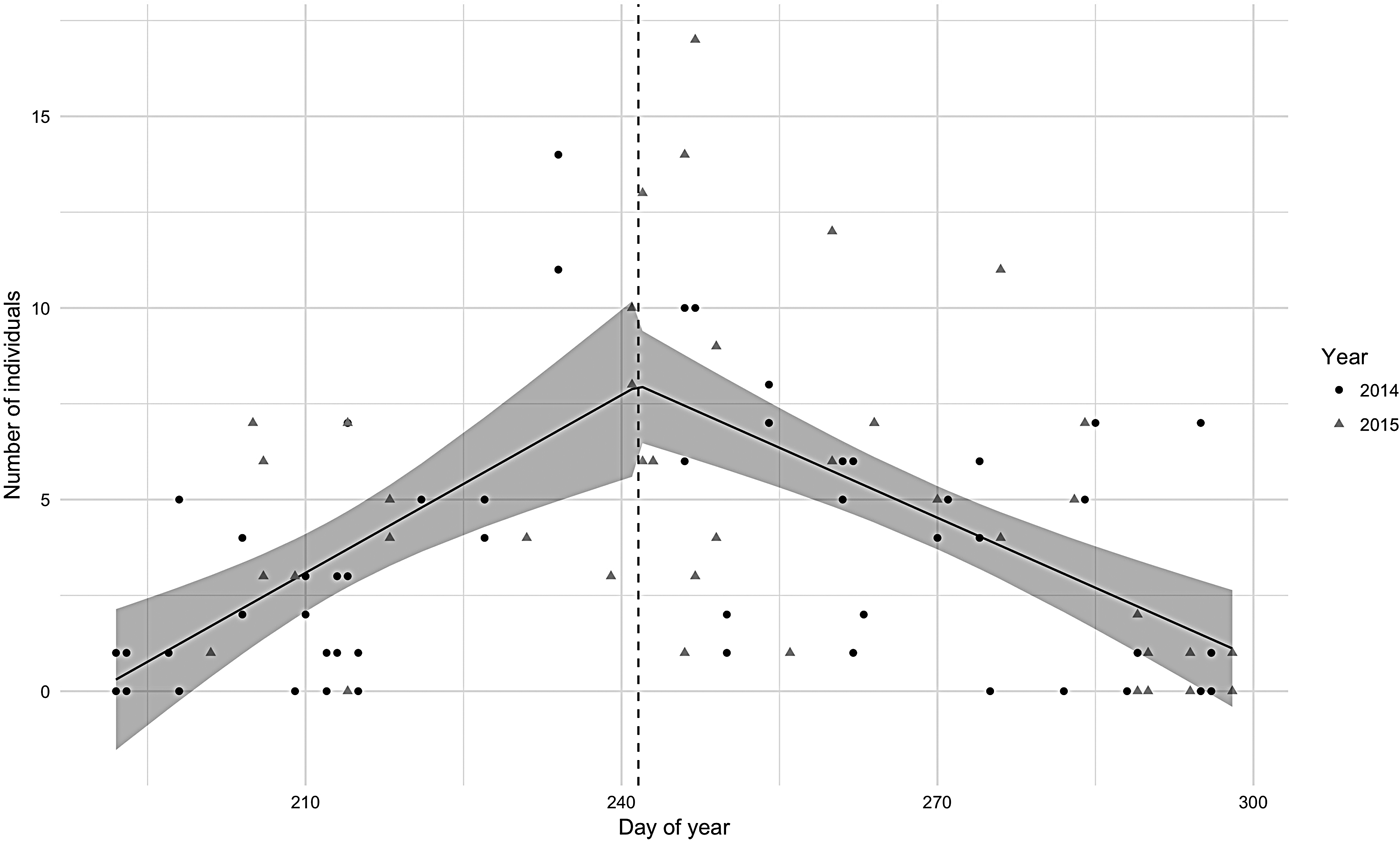
Fig. 2. Hourly number of humpback whales observed in Serra Grande (Bahia state, Brazil) along the Julian days in 2014 (dots) and 2015 (triangles) with the segmented regression 95% confidence interval model showed in grey.
In 2015, the mean number of individuals per hour (N = 5.12, SD = 4.18) was significantly greater (W = 809, P < 0.05) than in 2014 (N = 3.44, SD = 3.35). This difference was due to the higher number of adults observed per hour (W = 813, P < 0.05) in 2015 (N = 4.68, SD = 3.74) when compared with adult numbers in 2014 (N = 3.19, SD = 3.13). The number of calves did not change significantly between years (W = 949, P = 0.24) although the absolute counts were higher in 2015 (N = 0.44, SD = 0.8; N = 0.25, SD = 0.52 in 2014).
Based on GLM, adult number was affected by the year (P < 0.05) and lunar phase. The full moon was considered as the baseline lunar phase in the model and there were significantly fewer adults in the area during the new moon (P < 0.001) and first quarter (P < 0.01) but no significant difference was verified during the last quarter (P = 0.33). The interaction between Julian day and year also influenced the adult numbers (P < 0.05); different peaks in adult abundance occurred between the years and a sharper decrease in the adult numbers beginning in the end of September was observed for 2015 when compared with 2014. Sea state did not affect adult humpback whale abundance (Table 3).
Table 3. Parameter and P-values estimated using a Generalized Linear Model with Poisson distribution that explained adult relative abundance observed from a land-based observation station in 2014 and 2015 in Serra Grande (Bahia state, Brazil). Predictor variables were: year, Julian day, sea state (Beaufort), lunar phase and the interaction between Julian day and year.
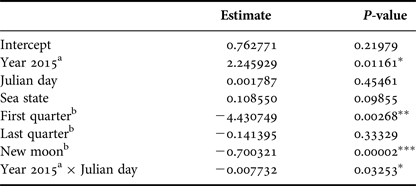
P-values: *P < 0.05, **P < 0.01, ***P < 0.001.
a Difference from 2014.
b Difference from full moon.
Number of calves was positively affected by the Julian day (P < 0.01) and by the number of adults (P < 0.001). Year, lunar phase and sea state did not affect the number of calves (Table 4).
Table 4. Parameter and P-values estimated using a Generalized Linear Model with Poisson distribution that explained calf relative abundance observed from a land-based observation station in 2014 and 2015 in Serra Grande. Predictor variables were: year, Julian day, sea state (Beaufort), lunar phase, number of adults and the interaction between Julian day and year.
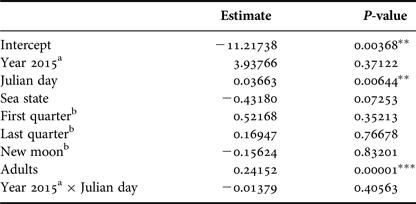
P-values: *P < 0.05, **P < 0.01, ***P < 0.001.
a Difference from 2014.
b Difference from full moon.
Habitat use
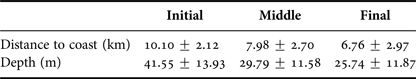
Depth increases as a function of distance from the coast and beyond 11 km this effect is higher (Figure 3). The majority of humpback whale groups (90.3%) were sighted in waters of less than 50 m depth and 67.6% up to 10 km away from the coast. Mean distance to coast gradually decreased through the season (F = 22.22, d.f. = 139, P < 0.001; Table 5, Figure 4) and was significantly different between initial and middle periods (P < 0.01) and between initial and final periods (P < 0.001), but not significant between middle and final periods (P = 0.07). Similarly, mean depth values in which whales occurred varied among periods (F = 23.08, d.f. = 139, P < 0.001; Table 5), decreasing as the season progressed, being significantly different between the initial and middle periods (P < 0.001) and between initial and final periods (P < 0.001). No significant differences in mean depth of humpback whale sightings were observed between middle and final periods of the season (P = 0.23).
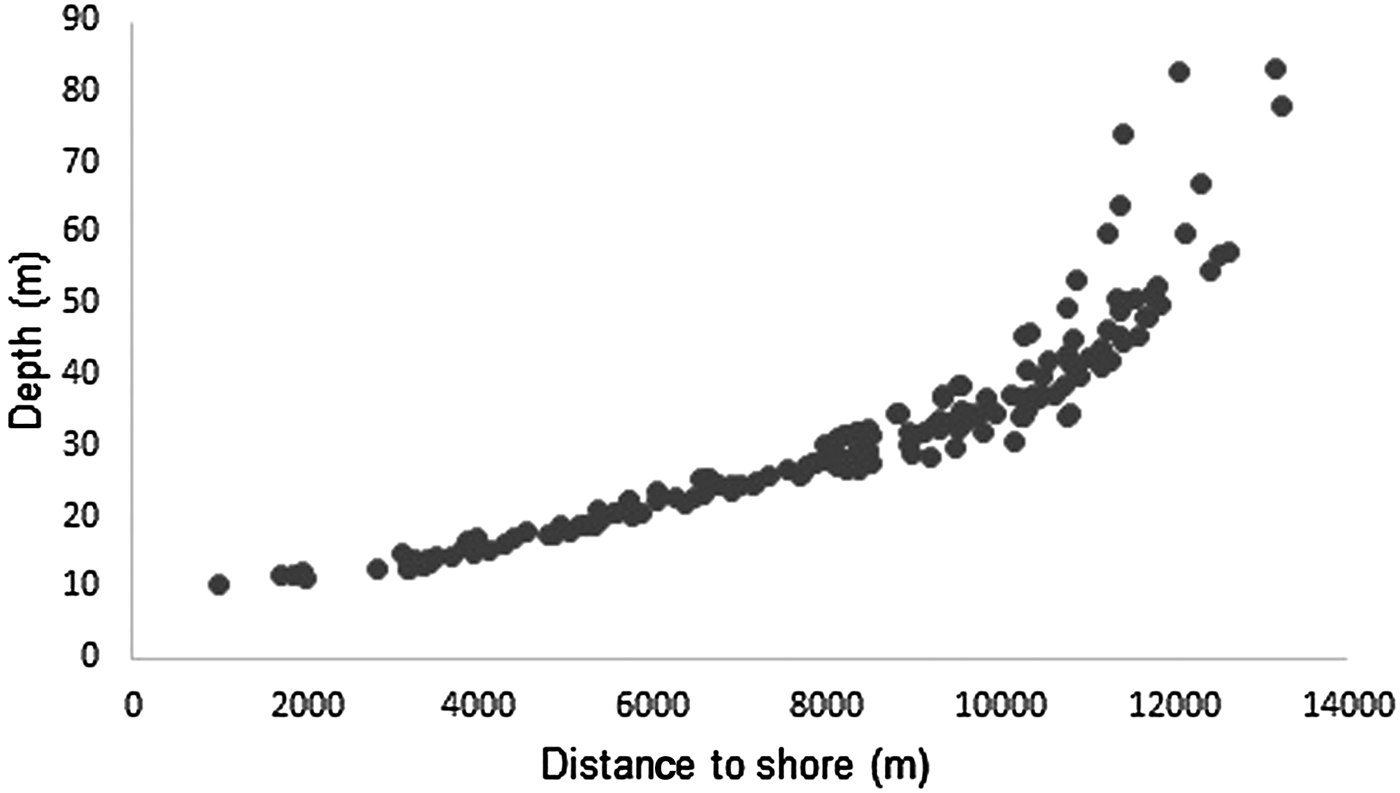
Fig. 3. Relationship between distance to coast and depth of humpback whale groups sighted from a land-based observation station in 2014 and 2015 in Serra Grande, Bahia state, Brazil.
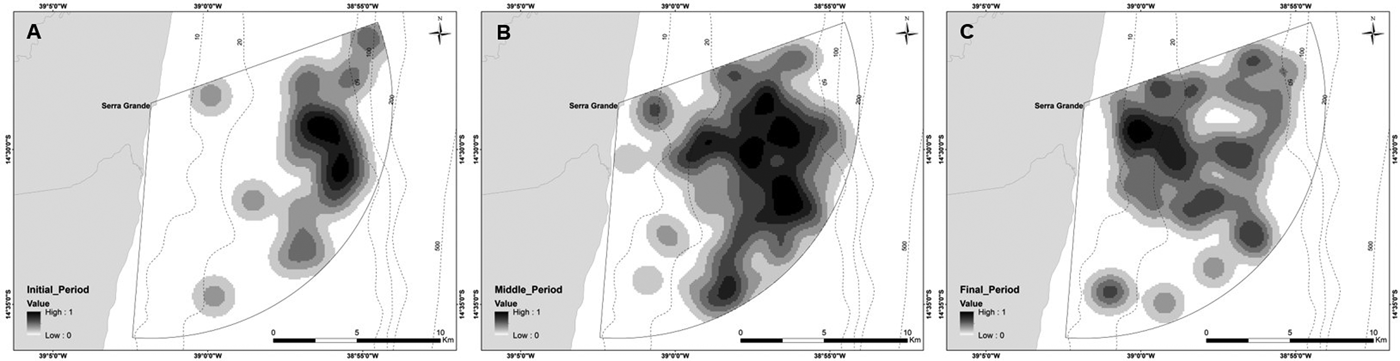
Fig. 4. Kernel density maps of all groups of humpback whales sighted in 2014 and 2015 from a land-based observation station at Serra Grande (Bahia state, Brazil) divided by periods of the season: (A) initial; (B) middle; (C) final.
Table 5. Descriptive statistics (mean ± SD) for distance to coast and depth values of humpback whale groups sighted from a land-based observation station in Serra Grande (Bahia state, Brazil) per periods of the season (initial, middle, final) in the years 2014 and 2015.
Mean values for distance to coast were significantly greater (t = 5.2019, d.f. = 39.588, P < 0.001) for groups without calves (8.78 ± 2.33 km) than for groups with calves (5.58 ± 3.19 km). Within groups with calves, the distances where each group type were sighted were significantly different (F = 7.161, d.f. = 29, P < 0.05). Groups of MOC were sighted significantly closer to the coast than MOCE/+ (P < 0.05) but no significant differences between MOC and MOCE (P = 0.08) were found (Table 6).
Table 6. Mean and SD of distance from coast and depth of the humpback whale groups with calves observed in 2014 and 2015 in Serra Grande, Brazil (MOC = mother and calf, MOCE = mother and calf and one escort, MOCE/+ = mother and calf and two or more escorts).

We found significant differences in mean depth for groups with and without calves (t = 4.3084, d.f. = 47.079, P < 0.001). Groups with calves were in shallower waters (22.38 ± 12.67 m) than groups without calves (33.41 ± 12.28 m). Also, there were significant differences in the mean depth of sightings of the different types of groups with calves (F = 6.2516, num. d.f. = 1.000, denom. d.f. 14.625, P < 0.05): MOC were sighted in shallower waters than MOCE (P < 0.05) and MOCE/+ (P < 0.05) (Table 6).
DISCUSSION
To our knowledge, this is the first study describing the habitat use patterns of humpback whales in Serra Grande coastal low latitudes. Descriptions of baseline habitat use patterns in coastal areas while there is a low level of human disturbance are essential for humpback whale conservation, in particular where overlap with human activities may occur in the future, such as the construction of a new port in the region (BAMIN, 2011).
Group characteristics
Mean humpback whale group size in Serra Grande was similar to that observed in other calving areas such as Abrolhos in Brazil (Martins et al., Reference Martins, Morete, Engel, Freitas, Secchi and Kinas2001), Ecuador (Scheidat et al., Reference Scheidat, Castro, Denkinger, González and Adelung2000; Félix & Haase, Reference Félix and Haase2001) and the east coast of Australia (Franklin et al., Reference Franklin, Franklin, Brooks, Harrison, Baverstock and Clapham2011). We did not observe variation in group sizes through the season nor between the two sampled years as also occurred in Abrolhos (Morete et al., Reference Morete, Bisi and Rosso2007). Nonetheless, in Hawai'i (Baker & Herman, Reference Baker and Herman1984) and in Ecuador (Félix & Haase, Reference Félix and Haase2001), group sizes tend to increase as the season progresses due to an increase in mature male densities searching for receptive females in competitive groups. Each population might have different social strategies depending on site-specific contexts or even on culture.
The proportions relating to group composition observed are identical to the areas surveyed north of Serra Grande (Lunardi et al., Reference Lunardi, Engel and Macedo2008; Rossi-Santos et al., Reference Rossi-Santos, Neto, Baracho, Cipolotti, Marcovaldi and Engel2008). The proportion of groups with calves is much smaller in Serra Grande (21%) than around the Abrolhos Archipelago (48%) (Morete et al., Reference Morete, Bisi and Rosso2007), which is within the main calving ground for the population that migrates to Brazil. Also, the proportion of mother-calf pairs escorted by a single adult (MOCE) is much higher in Abrolhos (Morete et al., Reference Morete, Bisi and Rosso2007), and may be related to the geomorphological characteristics as further discussed.
Relative abundance patterns
It is not surprising that the number of whales sighted has increased from 2014 to 2015 since the Population Stock A has risen in recent years (Andriolo et al., Reference Andriolo, Kinas, Engel, Martins and Rufino2010; Bortolotto et al., Reference Bortolotto, Danilewicz, Andriolo, Secchi and Zerbini2016).
The peak of the season varied between years; in 2015, it was 12 days later than in 2014. Nevertheless, there was a marked decrease in the number of whales observed in late September in 2015. These temporal fluctuations in relative abundance have been observed in other humpback whale reproductive areas (Baker & Herman, Reference Baker and Herman1981; Corkeron et al., Reference Corkeron, Brown, Slade and Bryden1994; Mattila et al., Reference Mattila, Clapham, Vásquez and Bowman1994; Frankel & Clark, Reference Frankel and Clark2002; Morete et al., Reference Morete, Bisi, Pace and Rosso2008) and may be related to migratory triggers in low and high latitudes. Dawbin (Reference Dawbin and Norris1966) suggests that photoperiod plays a role in migratory timing in high latitudes. Sea surface temperature (Nishiwaki, Reference Nishiwaki1959) and food resource availability in the previous summer (Craig et al., Reference Craig, Herman, Gabriele and Pack2003) are thought to be the most important factors that trigger humpback whale migration to the feeding grounds (Abras, Reference Abras2014). The fat layer accumulated from summer feeding prior to migration to low latitudes would limit the permanence of individuals in their reproductive areas (Craig et al., Reference Craig, Herman, Gabriele and Pack2003). In Brazilian waters, the ‘El Ni ñ o’ phenomenon caused an increase in the sea surface temperature in 2015 (NOAA, 2016). The temperature rise started in August 2015 and could have affected the timing of return of humpback whales to Antarctica, explaining the sharp decrease in abundance after the peak of the 2015 season.
Highest adult abundance coincided with full and last quarter lunar phases. Lunar phase affects when males are more likely to sing during the day in Abrolhos (Sousa-Lima & Clark, Reference Sousa-Lima and Clark2008). In Angola, lunar phase affects the relative abundance of singers (Cerchio et al., Reference Cerchio, Strindberg, Collins, Bennett and Rosenbaum2014), the authors detecting fewer singers during full moon than at new moon. Humpback whale singers are often characterized by slow-moving individuals (Tyack & Whitehead, Reference Tyack and Whitehead1983; Spitz et al., Reference Spitz, Herman, Pack and Deakos2002; Noad & Cato, Reference Noad and Cato2007) and thus less likely to be detected by visual surveys (Noad & Cato, Reference Noad and Cato2007) when compared with passive acoustic monitoring (Frankel et al., Reference Frankel, Clark, Herman and Gabriele1995; Noad & Cato, Reference Noad and Cato2007). One of the possible reasons for differences in number of whales counted by us during the new moon could be that the behaviour of singing males makes them harder to be detected from a land-based observation station, and we might have underestimated the number of adults by missing singers during the new moon. Alternatively, maybe the song keeps other acoustic competitors further away, consequently leading to the presence of a smaller number of individuals, or the low densities lead to males singing more to attract females. Studies on the abundance of singers in the area may elucidate these findings.
The number of calves increased throughout the season, the same pattern observed in Abrolhos, where the number of calves varied with the number of adults and the Julian day (Morete et al., Reference Morete, Pace, Martins, Freitas and Engel2003). Surprisingly, the number of calves did not increase from 2014 to 2015, differing from what was observed for adults. Morete et al. (Reference Morete, Bisi, Pace and Rosso2008) surveyed the Abrolhos Archipelago for 7 years, and noted an increase in the number of adults but the number of calves remained the same, and only increased significantly in the last year sampled. During the same years, Morete et al. (Reference Morete, Bisi and Rosso2007) did not find evidence that the number of adults in the groups observed in Abrolhos increased over the years, concluding that females with calves could be using different areas or that the number of calves would be the result of falling birth rates. Clapham (Reference Clapham1996) suggests that reproductive rates may be affected by food availability, as was also proposed by Seyboth et al. (Reference Seyboth, Groch, Dalla Rosa, Reid, Flores and Secchi2016) for reproductive success of the southern right whales. Therefore, the constant number of calves observed between 2014 and 2015 could be a result of lower food availability in the 2014/2015 summer feeding ground or a change in preferred calving area by females in 2015.
Bad weather conditions that result in high sea state levels (Beaufort scale) may reduce whale detection probability (Corkeron et al., Reference Corkeron, Brown, Slade and Bryden1994). Nonetheless, when observations were restricted to sea state up to Beaufort level 4, the number of adults and the number of calves sighted in Serra Grande were not affected. Smultea (Reference Smultea1994) had a limit for data collection of up to Beaufort 3 and also did not find any effect of sea state on detection rates. Frankel & Clark (Reference Frankel and Clark2002) found that the sighting rate was negatively affected by the sea state when working up to Beaufort 6, and noted this effect particularly applied in offshore areas. There is a trade-off between the amount of data collected (considering higher sea state levels) and quality and reliability of sightings (missed detections).
Social organization and habitat use
Distribution of whales varied throughout the season, with groups using waters closer to the coast as the season progresses, as also observed in Western Australia by Jenner et al. (Reference Jenner, Jenner and McCabe2001), who suggested that the migratory route from the feeding areas to the calving ground would be further away from the coast and the path back to the feeding ground would be closer to the coast. The same pattern may be occurring off Serra Grande. Also, another reason that could explain this approximation is the spatial segregation of groups with and without calves that was identified in Serra Grande. The increase in the number of calves after the peak of the season may have caused the groups to stay closer to the coast as the season progressed.
Coastal areas such as Serra Grande, where the shelf break is closer to shore and depth changes abruptly, lead to more concentration of mother and calf groups than areas where depth varies gradually, such as off islands and archipelagos. In Serra Grande, the difference in mean depth between the sightings of groups with and without calves is around 10 m, and in Abrolhos is smaller than 5 m (Martins et al., Reference Martins, Morete, Engel, Freitas, Secchi and Kinas2001), where mean depth is 30 m, perhaps allowing escorts to have easier access to mothers and calves. Different habitat conditions across the range of humpback whales in Brazil may lead to differences in habitat use and social organization as observed in other populations (Félix & Botero-Acosta, Reference Félix and Botero-Acosta2011).
Groups with calves occupying shallower waters closer to shore are considered a social strategy (Ersts & Rosenbaum, Reference Ersts and Rosenbaum2003). Mothers could be protecting their calves against harassment from males trying to mate with them (Smultea, Reference Smultea1994), which may cause injury to the newly born calves (Baker & Herman, Reference Baker and Herman1984) as well as higher energy costs for both mother and calves (Cartwright & Sullivan, Reference Cartwright and Sullivan2009; Craig et al., Reference Craig, Herman, Pack and Waterman2014). Parental care could also explain why mothers remain closer to shore in shallow waters where there are fewer predators (Smultea, Reference Smultea1994), less turbulence (Whitehead & Moore, Reference Whitehead and Moore1982), and shallower depth, limiting the approach and manoeuvre of males (Ersts & Rosenbaum, Reference Ersts and Rosenbaum2003; Bruce et al., Reference Bruce, Albright, Sheehan and Blewitt2014). However, females with calves may allow the presence of an escort during transit in areas where they would be exposed to deeper, less protected waters (Ersts & Rosenbaum, Reference Ersts and Rosenbaum2003). An escort may offer protection to the mother-calf pair (Herman & Antinoja, Reference Herman and Antinoja1977), which was evidenced by the observations of escorts defending calves from predator attacks (Pitman et al., Reference Pitman, Totterdell, Fearnbach, Ballance, Durban and Kemps2015), acting as bodyguards (Mesnick, Reference Mesnick1996; Wilson & Mesnick, Reference Wilson, Mesnick and Gowaty1997; Cartwright & Sullivan, Reference Cartwright and Sullivan2009), or even protection from other males attempting to mate.
Groups with calves escorted by adults occur in deeper waters (Betancourt et al., Reference Betancourt, Herrera-Moreno and Beddall2012; MacKay et al., Reference MacKay, Würsig, Bacon and Selwyn2016) and in Serra Grande, as the number of escorts increases, the distances from shore increase. Craig et al. (Reference Craig, Herman, Pack and Waterman2014) and Félix & Botero-Acosta (Reference Félix and Botero-Acosta2011) observed similar results and suggested that water depth not only limits the association of escorts to mother-calf pairs but would also limit the movement of courting males. Larger groups with calves would comprised inexperienced mothers that are unable to avoid being joined by multiple escorts (Elwen & Best, Reference Elwen and Best2004). Distance to coast and water depth are environmental factors that explain the distribution of humpback whale groups. Thus, our results support the interaction between environmental constraints and social organization proposed for the species (Félix & Botero-Acosta, Reference Félix and Botero-Acosta2011).
CONCLUSION
Serra Grande has a lower percentage of groups with calves compared with Abrolhos, but this percentage is comparable to other reproductive grounds (Ersts & Rosenbaum, Reference Ersts and Rosenbaum2003; Rasmussen et al., Reference Rasmussen, Calambokidis and Steiger2011). Habitat use patterns also support the notion that this is a calving area because of the typical increase in the abundance of calves as the season progresses. As populations recover, the presence of humpback whales in other low latitude areas tends to expand. The importance of Serra Grande as a calving area will probably increase given the uniqueness of this site in having the shortest distance to the shelf break in Brazil (IBGE, 2011; Prates et al., Reference Prates, Gonçalves and Rosa2012), allowing humpback whales to concentrate very close to the coast. It is noteworthy that despite being a small-scale study, we observed the same pattern as found in larger scale studies (Zerbini et al., Reference Zerbini, Andriolo, Rocha, Simões-Lopes, Siciliano, Pizzorno, Waite, DeMaster and VanBlaricom2004), reinforcing this general pattern for humpback whales off Brazil. Land-based platforms in high altitude stations are cost effective and representative of habitat use patterns. These local efforts throughout the area of occurrence may reveal which environmental factors better explain humpback whale distribution and abundance on a larger scale. Decision making for the creation of protected areas (Andriolo et al., Reference Andriolo, Kinas, Engel, Martins and Rufino2010) and the implementation of human activities at sea will be supported by robust knowledge of site-specific abundance patterns, avoiding potential problems such as collisions with vessels (Redfern et al., Reference Redfern, Mckenna, Moore, Calambokidis, Deangelis, Becker, Barlow, Forney, Fiedler and Chivers2013) and site abandonment (Jones & Swartz, Reference Jones, Swartz, Perrin, Würsig and Thewissen2009). Additionally, the general public can profit from touristic activities by experiencing land- or boat-based whale watching.
ACKNOWLEDGEMENTS
Special thanks to the following field assistants: Erica Lopes, Evelyn Fróes, Juliede Nonato, Luana Pini, Mariana Campêlo, Stella Tomás and Winnie Silva, and for the logistical support at the land-based observation station provided by Giulio Lombardi, Davi Santiago, Mr Nelson Cangirana and Mr Raimundo Gomes. We would like to thank Artur Andriolo, Cristiane C.A. Martins, Luciano Dalla Rosa and Yvonnick Le Pendu for their valuable comments on the text.
FINANCIAL SUPPORT
We thank the Coordination for the Improvement of Higher Education Personnel (CAPES) for the PhD scholarship granted to M.I.C.G. Financial support for fieldwork was provided by the Universidade Estadual de Santa Cruz (UESC) to J.E.B. and by Cetacean Society International (2014 and 2015) to M.I.C.G. M.E.M. is part of Projeto Baleia Jubarte which is sponsored by Petróleo Brasileiro S.A. (Petrobras). G.H.C. received financial support from the São Paulo Research Foundation (FAPESP #14292-9).












