Published online by Cambridge University Press: 03 December 2014
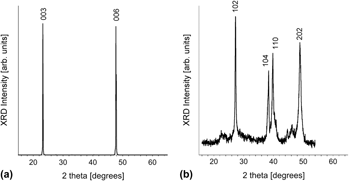
Using galvanostatic pulse deposition, we studied the factors influencing the quality of electroformed Bi1–xSbx nanowires with respect to composition, crystallinity, and preferred orientation for high thermoelectric performance. Two nonaqueous baths with different Sb salts were investigated. The Sb salts used played a major role in both crystalline quality and preferred orientations. Nanowire arrays electroformed using an SbI3-based chemistry were polycrystalline with no preferred orientation, whereas arrays electroformed from an SbCl3-based chemistry were strongly crystallographically textured with the desired trigonal orientation for optimal thermoelectric performance. From the SbCl3 bath, the electroformed nanowire arrays were optimized to have nanocompositional uniformity, with a nearly constant composition along the nanowire length. Nanowires harvested from the center of the array had an average composition of Bi0.75Sb0.25. However, the nanowire compositions were slightly enriched in Sb in a small region near the edges of the array, with the composition approaching Bi0.70Sb0.30.