Article contents
Synthesis, growth mechanism, and morphology control of LiFe1/3Mn1/3Co1/3PO4 via a microwave-assisted hydrothermal method
Published online by Cambridge University Press: 23 March 2015
Abstract
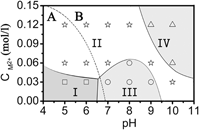
LiFe1/3Mn1/3Co1/3PO4 (LFMC) has been synthesized by a microwave-assisted hydrothermal technique. During the crystal growth, two evolutionary routes coexist and compete with each other after the nuclei have been stably formed. One of them is the continuous growth of single particles and the other one is agglomeration. The size and morphology of the products are determined by the interplay of the two competing routes. The growth morphology is quantitatively analyzed from first principle calculations. A phase diagram is constructed, which guides to control the morphology by adjusting CM and pH. Static magnetic properties imply long range antiferromagnetic order below TN = 39 K and a paramagnetic Curie–Weiss-like behavior with θ = 75 K and peff = 5.51 μB at high temperatures. Cyclic voltammetry shows two distinct peaks corresponding to the Fe2+/Fe3+ and Co2+/Co3+ redox couples, respectively, whereas the Mn2+/Mn3+ redox couple is not observed due to its sluggish kinetics induced by the Jahn–Teller effect of Mn3+.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2015
References
REFERENCES
- 3
- Cited by


