Article contents
Spectroscopic studies of nucleic acid additions during seed-mediated growth of gold nanoparticles
Published online by Cambridge University Press: 06 February 2015
Abstract
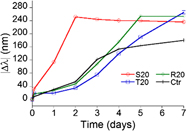
The effect of adding nucleic acids to gold seeds during the growth stage of either nanospheres or nanorods was investigated using UV–Vis spectroscopy to reveal any oligonucleotide base or structure-specific effects on nanoparticle growth kinetics or plasmonic signatures. Spectral data indicate that the presence of DNA duplexes during seed aging drastically accelerated nanosphere growth while the addition of single-stranded polyadenine at any point during seed aging induces nanosphere aggregation. For seeds added to a gold nanorod growth solution, single-stranded polythymine induces a modest blue shift in the longitudinal peak wave length. Moreover, a particular sequence comprised of 50% thymine bases was found to induce a faster, more dramatic blue shift in the longitudinal peak wave length compared to any of the homopolymer incubation cases. Monomeric forms of the nucleic acids, however, do not yield discernable spectral differences in any of the gold suspensions studied.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2014
References
REFERENCES
- 3
- Cited by


