Article contents
Self-assembly synthesis and mechanism investigation of branched core–shell hybrids of tin nanowires and carbon nanotubes
Published online by Cambridge University Press: 27 November 2012
Abstract
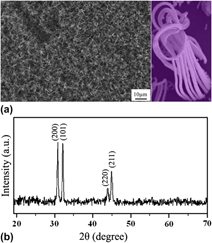
Branched core–shell hybrids of tin nanowires and carbon nanotubes have been successfully obtained on silicon substrate via a self-assembly process by chemical vapor deposition. Structure characterization unveiled that the nanostructures are the hybrids of branched single-crystalline β-Sn nanowires coated with amorphous carbon nanotubes. Detailed investigation demonstrates that the amount of introduced ethylene plays a crucial role in triggering the morphology change of the product from freestanding core–shell hybrids to branched hybrids accompanying with a thickness and surface morphology change of carbon shell. Architecture of the branched core–shell hybrids has been categorized and the mechanism has been discussed. This kind of branched hybrids may find great potential applications in building multipath nanoelectronic components, lithium-ion battery electrodes, and enhanced superconducting nanodevices as well.
Keywords
- Type
- Articles
- Information
- Journal of Materials Research , Volume 28 , Issue 7: Focus Issue: De Novo Carbon Nanomaterials: Opportunities and Challenges in a Flat World , 14 April 2013 , pp. 969 - 975
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 3
- Cited by


