Published online by Cambridge University Press: 30 August 2017
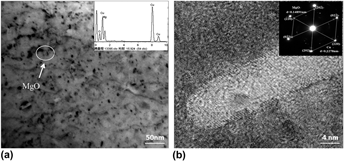
MgO/Cu composites containing a 1.0% volume fraction of MgO particles were prepared by internal oxidation and powder metallurgy, respectively. The interfacial bonding state between the MgO particles and Cu matrix was characterized by scanning electron microscopy and transmission electron microscopy. The effect of the MgOp/Cu interfacial bonding state on the arc erosion resistance of the MgO/Cu composites was investigated, and the arc erosion resistance was examined using a JF04C electrical composite testing system. The results indicate that the 1.0 vol% MgO/Cu composite with a semicoherent MgOp/Cu interface experiences a lower arc erosion rate and smaller fluctuations of arcing energy than those of the 1.0 vol% MgO/Cu composite with an incoherent MgOp/Cu interface. Erosion morphology observations further indicate that a solid to liquid phase transformation occurs under arcing and MgO particles dispersed in the molten copper both prevent the copper matrix from splashing and enhance the arc erosion resistance of the MgO/Cu composites. While the shallow electric erosion pits are distributed uniformly on the arc surface of the MgO/Cu composites with a semicoherent interface, the MgO/Cu composite with an incoherent interface has deep and uneven pits on its arc surface, characterized by large electric erosion molten droplets.
Contributing Editor: Jürgen Eckert