Article contents
Interactive formation of Cu-rich precipitate, reverted austenite, and alloyed carbide during partial austenite reversion treatment for high-strength low-alloy steel
Published online by Cambridge University Press: 02 May 2017
Abstract
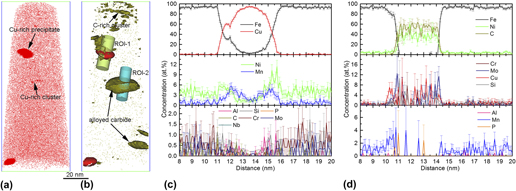
We address the competitive precipitation and coprecipitation of three types of secondary phases, i.e., Cu-rich precipitates (CRPs), reverted austenite (RA), and alloyed carbide, in a high-strength low-alloy steel with austenite reversion treatment at 675 °C by using electron back-scatter diffraction, transmission electron microscopy, and atom probe tomography. There is a strong competitive diffusion of Ni and Cu participating in austenite reversion and Cu precipitation with the fact that no CRPs are detected in and around the RA. Meanwhile, there is also a strong competitive diffusion of austenite stabilizing element Ni and carbide-forming elements Cr and Mo into the pre-existing C-rich zone, leading to the formation of nonequilibrium alloyed carbide deviating from the stoichiometric composition. On the other hand, the alloyed carbide and CRPs provide constituent elements for each other and make the coprecipitation thermodynamically favorable. The knowledge on the interactive formation of these three features provides versatile access to tailor the distributional morphology of CRPs, RA, and alloyed carbide via a multistage heat treatment and thus realize their beneficial effect on strength and toughness.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: Jürgen Eckert
References
REFERENCES
- 4
- Cited by



