Article contents
Influence of Mo content on the phase evolution and corrosion behavior of model Fe–9Cr–xMo (x = 5, 7, and 9 wt%) alloys
Published online by Cambridge University Press: 05 June 2015
Abstract
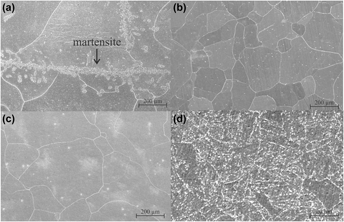
Model Fe–9Cr–xMo alloys with high Mo content alloys were cast to investigate the effect of Mo on the phase evolution and properties of the alloys. The Mo contents in the alloys were 5, 7, and 9 wt%, while the Cr content was held constant at 9 wt%. For comparison, a commercial A213T 9-P9 (Fe–9Cr–1Mo) alloy, widely used in the petrochemical industry, was also investigated. The alloys were heat-treated at temperatures between 450 and 650 °C and characterized by scanning electron microscopy, x-ray dispersive energy spectroscopy, and electron backscatter diffraction. Potentiodynamic linear polarization technique was used to evaluate the corrosion of the alloys in a 0.5 mol L−1 H2SO4 solution containing 0.1 mol L−1 NaCl. The results showed that the corrosion resistance of the alloys was affected by precipitation of the µ phase and that the Mo content in excess of 5% is deleterious to the corrosion resistance of the Fe–Cr–Mo alloys.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2015
References
REFERENCES
- 6
- Cited by


