Published online by Cambridge University Press: 18 May 2017
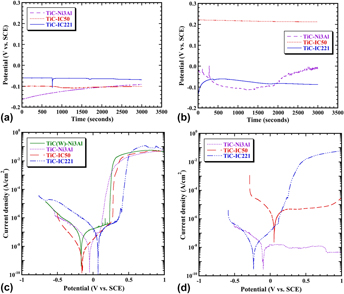
The aqueous corrosion resistance of TiC–Ni3Al based cermets was examined with the specific aims of assessing the influence of both the raw materials and the test methods, using a 3.5 wt% NaCl containing solution. The effects of W contamination in the ceramic phase was investigated using a single-phase ceramic, with and without W, and with a stoichiometric intermetallic Ni3Al binder. The influence of the electrolyte O2 content was examined for TiC cermets with 30 vol% binder, for both the stoichiometric composition and sub-stoichiometric variants, containing either Zr and B (alloy IC50) or Zr, B and Cr (alloy IC221) additions. Electrochemical measurements were matched with chemical and microstructural analyses at various stages of oxidation, and the rate of material loss in combination with the corrosion mechanisms were identified. The effects of O2 concentration were most significant for the TiC based cermets with Ni3Al due to the diffusion controlled nature of the reaction.
Contributing Editor: Xiaowei Yin