Article contents
Electroforming of oxide-nanoparticle-reinforced copper-matrix composite
Published online by Cambridge University Press: 03 February 2015
Abstract
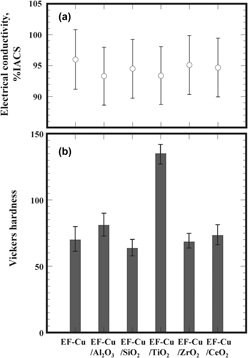
A method based on the electroforming technique has been proposed for the fabrication of nanoparticle-reinforced copper-matrix composites using an electrolyte of copper sulfate–sulfuric acid solution containing 1 cm3/L of the nanoparticles without surfactants. Of the tested nanoparticles such as Al2O3, SiO2, TiO2, ZrO2, and CeO2, whose sizes ranged from 10 to 30 nm, only TiO2 nanoparticles were successfully embedded in the copper matrix during electroforming, owing to their positive charge in the electrolyte solution. It should be noted that there was very little contamination in the copper matrix, because surfactants were absent during electroforming. Therefore, the electrical conductivity of the specimen that was electroformed in the electrolytes with TiO2 nanoparticles was not significantly different from that of pure copper. Nevertheless, the hardness, yield, and ultimate tensile strength were significantly improved by a small amount (0.3 mass%) of TiO2 nanoparticles primarily because of strengthening by Orowan mechanics. The electroforming process is thus a promising means to prepare copper-matrix composites with an excellent balance of electrical conductivity and mechanical strengths.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2015
References
REFERENCES
- 10
- Cited by


