Published online by Cambridge University Press: 28 September 2015
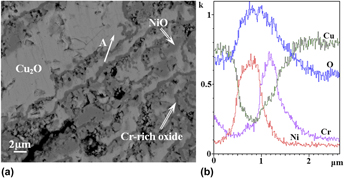
Cr is one of common alloying elements that improve the oxidation resistance of Cu. Its content and distribution are the two important factors that influence the oxidation resistance of alloys. In this paper, the cluster structure model of stable solid solutions was used for designing Cu–Cu12–[Crx/(12+x)Ni12/(12+x)]5 (x = 1, 2, 4, 6 or 8) alloys. The insoluble antioxidative Cr was uniformly distributed in the Cu matrix with the help of Ni element. The solid solution and precipitation of alloys were effectively controlled by changing the Cr/Ni ratio, and the effects of the distribution of alloying elements on the oxidation resistance of Cu alloy were discussed. The studies on isothermal oxidation at 850 °C show that element Cr of the Cu–Ni–Cr alloys designed using cluster model can be dispersed in the Cu matrix, and a continuous protective Cr-rich oxide layer can be formed when the Cr content dispersed in the alloys reaches 9.26 at% during isothermal oxidation. 1/2 of Cr in the Cu–Ni–Cr alloys is replaced by Fe, forming Cu–Ni–(Cr + Fe) alloys; oxide precipitates exhibit a columnar structure revealing the orientation of growth perpendicular to the matrix, thereby forming a lot of O diffusion channels and resulting in a sharp decline in its oxidation resistance. Therefore, the growth morphology of the oxide precipitates may significantly affect the oxidation resistance.
Contributing Editor: Yang-T. Cheng