Article contents
Annealing-induced lattice recovery in room-temperature xenon irradiated CeO2: X-ray diffraction and electron energy loss spectroscopy experiments
Published online by Cambridge University Press: 10 February 2015
Abstract
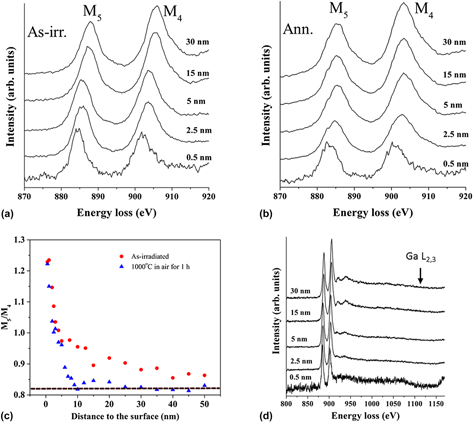
A systematic x-ray diffraction (XRD) study was performed on room-temperature Xe-irradiated and postirradiation annealed CeO2. Large scale XRD did not show any additional irradiation-induced phases upon irradiation. Depth profiling the CeO2 (111) diffraction peak over the 150 nm deep Xe-irradiated layer (400 keV, 1 × 1020 Xe/m2) by grazing incidence XRD indicated a lattice expansion at the irradiated layer. Postirradiation annealing (1 h at 1000 °C) in an oxygen-containing environment removed the observed XRD features. Electron energy loss spectroscopy (EELS) was performed for cross-sectional samples before and after postirradiation annealing. EELS showed that the Ce charge state changed from +4 to +3 at the CeO2 surface indicating the presence of O vacancies in both as-irradiated and annealed samples. EELS also indicated that the amount of O vacancies was reduced at the irradiated region by annealing. The experimental results are discussed based on electronic properties of CeO2, annihilation of oxygen vacancies, and evolution of irradiation damage.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2015
Footnotes
Contributing Editor: Khalid Hattar
References
REFERENCES
- 8
- Cited by


