Article contents
Silicon nanowire/polycaprolactone composites and their impact on stromal cell function
Published online by Cambridge University Press: 09 August 2012
Abstract
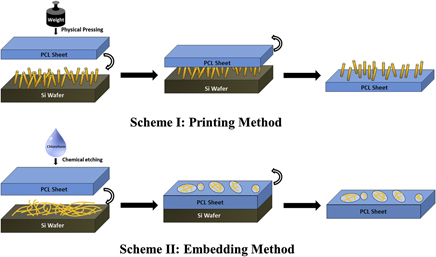
In this study, silicon nanowire (SiNW)/polycaprolactone composites with different surface topographies were fabricated by straightforward embedding or printing methods and their cytocompatibility was evaluated with a bone-relevant cell line derived from mouse stroma. The incorporation of biocompatible polymers with semiconducting SiNWs can ideally provide an enhanced environment to support proliferation and differentiation functions of bone cells. Cell/composite interactions were assessed with suitable assays including viability and alkaline phosphatase activity, while scanning electron microscopy characterization was used to study the morphology of cells grown on composites. Such results suggest that for nanowires in a vertical array, the presence of the polymer improves cellular attachment and overall viability relative to the nanowire-only system.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 28 , Issue 2: Focus Section: Silicon-based Nanoparticles for Biosensing and Biomedical Applications , 28 January 2013 , pp. 185 - 192
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 8
- Cited by


